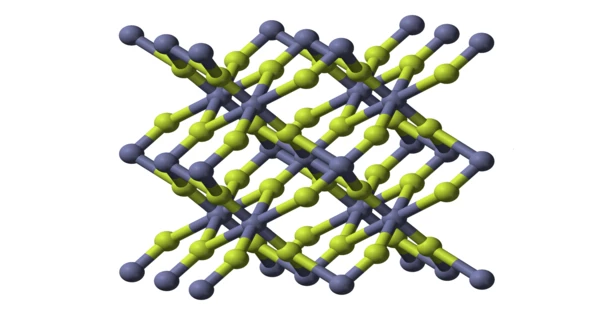
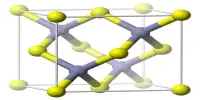
Zinc fluoride (ZnF2) is a chemical compound that is inorganic in nature. It takes the form of a white powder or crystalline mass. It can be found both anhydrous and tetrahydrate, ZnF2 • 4H2O. (rhombohedral crystal structure). It has a high melting point and a rutile structure with 6 coordinate zinc, indicating that it has a significant ionic character in its chemical bonding. It is not very soluble in water, unlike the other zinc halides, ZnCl2, ZnBr2, and ZnI2.
Zinc fluoride is used in the fluorination of organic compounds, the production of phosphors for fluorescent lights, the preservation of wood, electroplating baths, galvanizing steel, and the production of glazes and enamels in ceramic manufacturing.
Properties
- Chemical formula: ZnF2
- Molar mass: 103.406 g/mol (anhydrous); 175.45 g/mol (tetrahydrate)
- Appearance: white needles hygroscopic
- Density: 4.95 g/cm3 (anhydrous) 2.30 g/cm3 (tetrahydrate)
- Melting point: 872 °C (1,602 °F; 1,145 K) (anhydrous); 100 °C, decomposes (tetrahydrate)
- Boiling point: 1,500 °C (2,730 °F; 1,770 K) (anhydrous)
- Solubility in water: .000052 g/100 mL (anhydrous); 1.52 g/100 mL, 20 °C (tetrahydrate)
- Solubility: sparingly soluble in HCl, HNO3, ammonia

Preparation and reactions
Zinc fluoride can be synthesized several ways. Reaction of a fluoride salt with zinc chloride, to yield zinc fluoride and a chloride salt, in aqueous solution. The reaction of zinc metal with fluorine gas. Reaction of hydrofluoric acid with zinc, to yield hydrogen gas (H2) and zinc fluoride (ZnF2). Zinc fluoride can be hydrolysed by hot water to form the zinc hydroxyfluoride, Zn(OH)F.
Zinc fluoride may be prepared by heating zinc hydroxide or zinc carbonate with hydrogen fluoride:
Zn(OH)2 + 2HF → ZnF2 + 2H2O
ZnCO3 + 2HF → ZnF2 + CO2 + H2O
Also, it can be precipitated by adding a solution of sodium fluoride to that of zinc acetate:
(CH3COO)2Zn + 2NaF → ZnF2 + 2CH3COONa
Applications
Zinc Fluoride is a fluoride compound that is widely used in a variety of industries. It is used in the fluorination of organic compounds, as an additive in electrolytic aluminum, and in electroplating operations. It is also used in food preservation, the production of fluxes for metallurgy, oxygen sensitive applications for metal production, glass, enamels, fabrics, and plastics, as well as applications as a catalyst additive in oil refining, the production of pharmaceuticals, phosphors for electric lights, and the production of floor coverings.