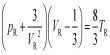Researchers are looking at ways to transfer more than one electron at a time, and some believe that the key is superatoms. Most modern technologies rely on semiconductors, elements where charges move from one group of atoms to another. This is usually done with electrons, where a single electron is passed between two atoms.
Superheroes are certainly a strange chemical creature. These are made up of clusters of atoms but act as one. Sometimes it can mimic the properties of different atoms or elements. What researchers are looking for is stability in moving charges and researchers have now come up with a new theoretical template for building superatoms. The strategy has been reported in Nature Communications.
Senior author Professor Shiv Khanna, from Virginia Commonwealth University, said in a statement, “The use of semiconductors in every sphere of life would be a significant social benefit to society that could significantly increase the Sup electron grant.”We have developed a new method to synthesize this type of metal-based superatoms.”
In the template, the researchers focus on the alkaline elements, which are included in the first column of the periodic table. These elements have a single electron on the outer shell which makes them quite reactive. It takes very little energy to remove these electrons but removing more than one from a single atom is a very powerful expense. And these are what make them good candidates for superatom treatment. A cluster of alkaline atoms can donate and receive different electrons with just a little energy.
Khanna said, “The possibility of having a building block that can take multiple charges or donate multiple charges could eventually find wide application in electronics.” These alkali superatoms have already been formed. The new template gives researchers a roadmap to build efficiently and can even help create real-life applications. An analysis by this team shows how such top notches can be protected and stabilized without understanding.
The team used computer simulations to test a specific class of these molecules called organic ligands. They combined aluminum clusters of boron, carbon, silicon, and phosphorus with organic ligands to show that the clusters could express electrons using less energy than any other alkali in the periodic table.