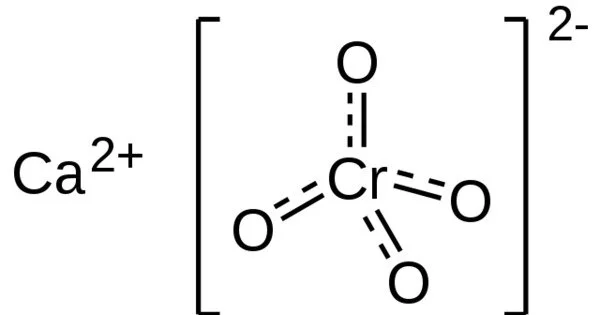
Calcium chromate, also known as the chromate salt of calcium, is an inorganic compound with the formula CaCrO4. It is a yellowish, crystalline inorganic compound that, when heated, emits toxic chromium fumes. CaCrO4•2H2O is a bright yellow solid that is normally found in the dihydrate form. Chromite is a very rare anhydrous mineral form that exists in nature.
Calcium chromate is a highly corrosive and oxidizing agent. This substance is primarily used in batteries as a corrosion inhibitor and depolarizer. The compound is used as a pigment on occasion, but this is limited due to the extremely toxic and carcinogenic nature of hexavalent chromium compounds such as chromate salts.
Properties
Calcium chromate is a yellow powder. It is slightly soluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to prevent its spread to the environment.
- Chemical formula: CaCrO4
- Molar mass: 156.072 g/mol
- Appearance: bright yellow powder
- Density: 3.12 g/cm3
- Melting point: 2,710 °C (4,910 °F; 2,980 K)
- Solubility in water: anhydrous 4.5 g/100 mL (0 °C); dehydrate 16.3 g/100mL (20 °C)
- Solubility: soluble in acid, practically insoluble in alcohol
- Crystal structure: monoclinic
Synthesis and reactions
Calcium chromate is formed from the salt metathesis reaction of sodium chromate and calcium chloride:
Na2CrO4 + CaCl2 → CaCrO4 + 2 NaCl
In aqueous solution the dihydrate is obtained, which loses water to afford the anhydrate at 200 °C.
It is an oxidizer, oxidizing organic compounds (e.g. alcohols) or reducing agents (e.g. metals) to the corresponding carbonyl compounds or metal oxides while the chromium(VI) centre in CaCrO4 is reduced to chromium(III).
Solid calcium chromate will react explosively with hydrazine. It will also burn violently if mixed with boron and ignited, thereby posing a fire hazard.
Uses
As part of the chromate conversion coating procedure, the compound is occasionally used as a yellow inorganic pigment or a corrosion inhibitor. It has also been used in chromium electroplating, photochemical processing, and industrial waste disposal.
It is also used in batteries as a depolarizer, as well as a metal coating and primer. It is also used in electroplating, photochemical processing, and the treatment of industrial waste.
Health hazards
It primarily affects the nose, throat, and lungs, causing ulcers, shortness of breath, bronchitis, pneumonia, and asthma-like allergic reactions, but it can also affect the gastrointestinal tract, liver, kidneys, and immune system.