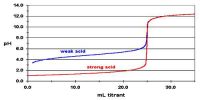
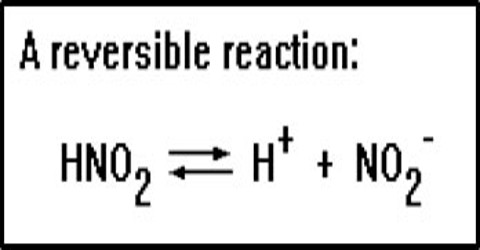A reversible reaction is a chemical reaction where the reactants form products that, in turn, react together to present the reactants back.
Explanation: When a fuel is burnt for cooking in a kitchen or for driving a motor car the reaction goes to completion until the supply of the fuel or oxygen is stopped. In kitchens using gas to produce the heat for cooking methane, CH4, is burnt. The reaction is:
CH4 (g) + 3 O2 (g) → CO2 (g) + 2 H2O (g)
Like all reactions in which a fuel is burnt this is a one way reaction because the products do not combine to form the reactants, and the reaction as written goes on until the gas or oxygen supply is stopped. For a given supply of one of the gases the reaction is complete.
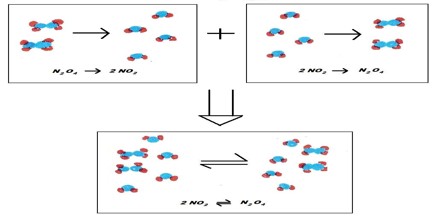
Many reactions, however, are reversible. This means that the reaction goes in both directions, i.e., if we start with the products, reactants will also start forming. Such reactions do not go to completion. Reactants are not entirely converted into products and at any time after start of the reaction, from the reactant side or the product side, one finds a mixture of the reactants and the products. Reaction from the reactant side is called the forward reaction and that from the product side is called the reverse reaction. The forward and reverse reactions together form a reversible reaction. The sign ‘↔’ between the reactants and the products indicate the type of a reaction. Consider the reaction,
N2O4 (g) ↔ 2 NO2 (g)
If some N2O4 (g), which is yellow al about 300C, is heated in a closed vessel it turns brown because of the formation of NO2 (g), which is brown. Again if some Brown NO2 (g) is taken in a closed vessel and it is cooled it starts turning yellow, because of the formation of N2O4 (g). So the above reaction is reversible. However if some N2O4 (g) or twice as much NO2 (g) is kept in a vessel at a constant temperature the colour gradually changes, but after a while it will be observed that the colour remains the same and does not change. This is because the proportion of the reactants and products remains unchanged. At this point the traction is said to have reached equilibrium. A chemical equilibrium in a system is the state of the system when there is no observable change in the concentration of the reactants and products.