Dynamic Chemical Equilibrium
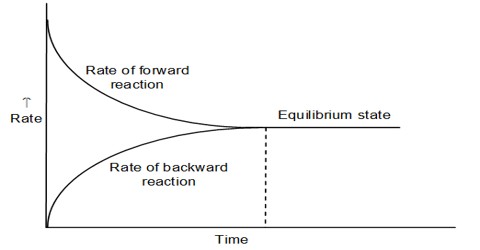
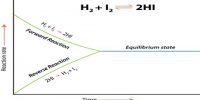
The constancy of the concentrations of the reactants and the products does not mean that at equilibrium no change is taking place. It has been shown b the use of radioactive isotopes and other means that at equilibrium reaction is still going on in both the forward and reverse directions. This is why chemical equilibria are said to be dynamic. A dynamic chemical equilibrium is one in which there is no net change of concentrations of the reactants and the products, although the reaction is proceeding in both directions. The concentrations of the reactants and products do not change because the rate of the forward and the rate of the reverse reaction are equal.
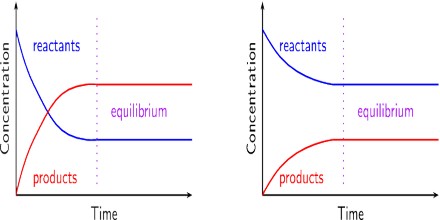
Fig: Concentration of the reactant and product as a function of time and attainment of Equilibrium.
Equilibrium systems could be divided into two groups homogeneous and heterogeneous. Homogeneous equilibria are those in which all the species (reactants and products) are in the same physical state. N2O4 (g) ↔ 2 NO2 (g) is an example of homogeneous equilibrium. Some other examples of homogeneous equilibrium are:
(1) H2 (g) + I2 (g) ↔ 2HI (g)
(2) PCl5 (g) ↔ PCl3 (g) + PCl2 (g)
(3) CH3COOH (l) + C2H5OH (l) ↔ CH3COOC2H5 (l) + H2O (g)
If one or more of the species in the equilibrium system is in a different physical state from the others then it is a heterogeneous equilibrium.
The equilibrium between liquid and vapour, is an example of heterogeneous equilibrium. Some other examples are,
(4) CaCO3 (s) ↔ CaO (s) + CO2 (g)
(5) 3Fe (s) + H2O (g) ↔ Fe3O4 (s) + 4H2 (g)
In all cases the equilibrium is dynamic in the sense that changes are continuously taking place in the forward and in the reverse directions although there is no net change in the state of the system.