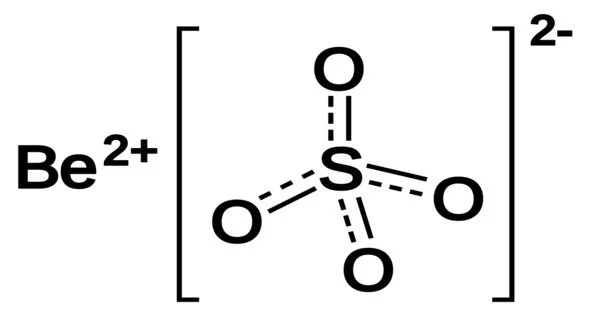
Beryllium sulfate is commonly found as a tetrahydrate, [Be(H2O)4]. SO4 is a crystalline white solid. It is a white, crystalline inorganic compound that when heated emits toxic fumes of beryllium oxides and sulfur oxides. Jons Jakob Berzelius discovered it for the first time in 1815. Beryllium sulfate can be made by treating an aqueous solution of many beryllium salts with sulfuric acid, then evaporating and crystallizing the solution. Heat the hydrated product to 400 °C to convert it to anhydrous salt.
Properties
- Chemical formula: BeSO4
- Molar mass: 105.075 g/mol (anhydrous); 177.136 g/mol (tetrahydrate)
- Appearance: white solid
- Odor: odorless
- Density: 2.44 g/cm3 (anhydrous); 1.71 g/cm3 (tetrahydrate)
- Melting point: 110 °C (230 °F; 383 K) (tetrahydrate, −2H2O); 400 °C (dihydrate, dehydr.)
- Boiling point: 2,500 °C (4,530 °F; 2,770 K) (anhydrate); 580 °C (tetrahydrate)
- Solubility in water: 36.2 g/100 mL (0 °C); 54.3 g/100 mL (60 °C)
- Solubility: insoluble in alcohol

Preparation
Beryllium Sulfate is a white crystalline solid. It was first isolated in 1815 by Jons Jakob Berzelius. Beryllium Sulfate may be prepared by treating an aqueous solution of any beryllium salt with sulfuric acid, followed by evaporation of the solution and crystallization.
Structure
The tetrahydrate has a tetrahedral Be(OH2)42+ unit and sulfate anions, according to X-ray crystallography. The number of water molecules that can be coordinated is determined by the small size of the Be2+ cation. The analogous magnesium salt, MgSO4•6H2O, on the other hand, contains an octahedral Mg(OH2)62+ unit. Vibrational spectroscopy confirmed the presence of the tetrahedral [Be(OH2)4]2+ ion in aqueous solutions of beryllium nitrate and beryllium chloride, as indicated by the totally symmetric BeO4 mode attt 531 cm-1. In beryllium sulfate, this band is absent, and the sulfate modes are perturbed. The evidence points to the existence of Be(OH2)3OSO3.
The anhydrous compound has a structure similar to that of berlinite. The structure contains alternating tetrahedrally coordinated Be and S and each oxygen is 2 coordinate (Be-O-S). The Be-O distance is 156 pm and the S-O distance is 150 pm.
Uses
Beryllium sulfate is primarily used to make beryllium oxide, which is used in high-tech ceramics. The neutron source in the discovery of nuclear fission was a mixture of beryllium and radium sulfate.
Safety
Inhaling its dust and fumes irritates the nose, throat, and lungs and can lead to pneumonitis. Prolonged beryllium exposure can cause berylliosis, a chronic beryllium disease that causes granuloma and fibrosis in the lungs. Beryllium has been linked to an increased risk of lung cancer.