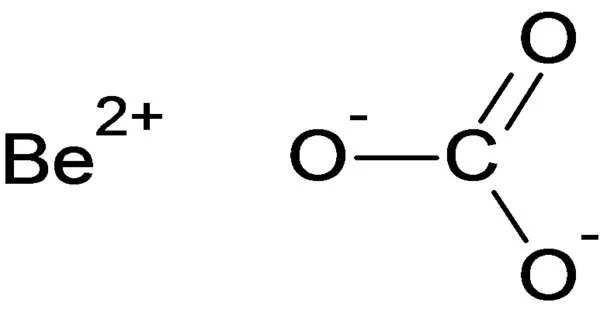Beryllium carbonate is a chemical compound with the formula BeCO3. Anhydrous, tetrahydrate, and basic beryllium carbonate are the three reported forms. According to reports, the anhydrous form is unstable, decomposing to BeO and carbon dioxide and necessitating storage under CO2. The tetrahydrate is said to form when CO2 is bubbled through a Be(OH)2 solution and is also said to be similarly unstable.
There is no evidence of naturally occurring pure beryllium carbonate. Niveolanite is the only known Be-rich carbonate mineral.
Properties
- Chemical formula: BeCO3
- Molar mass: 69.020 g·mol−1
- Melting point: 54 °C (129 °F; 327 K)
- Boiling point: 100 °C (212 °F; 373 K) decomposes
- Solubility in water: 0.36 g/100 mL
- Appearance: White Powder

Structures
There are three forms reported, anhydrous, a tetrahydrate, and basic beryllium carbonate. The anhydrous form is reported to be unstable, decomposing to BeO and carbon dioxide, and requiring storage under CO2. The tetrahydrate is said to be formed when CO2 is bubbled through a solution of Be(OH)2 and is also reported to be similarly unstable.
Preparation
Basic beryllium carbonate is a mixed salt, which can be prepared by the reaction of beryllium sulfate and ammonium carbonate, and contains both carbonate and hydroxide ions, with formula Be2CO3(OH)2. It is believed that in the older literature this is probably what was referred to as beryllium carbonate.
The increasing order of thermal stability of carbonates is as follows:
BeCO3 < MgCO3 < CaCO3 < SrCO3 < BaCO3
BeCO3 is very unstable mainly because of the enhanced stability of BeO over BeCO3.
Cations with high ionic potential have large power of polarization. In crystalline solid, the M2+ cation is surrounded by oxygen of CO32- ions. When ionic potential of cation (such as small Be2+) a partial covalent bond is setup between M2+ and O, as a result C-O bond becomes weak due to withdrawal of electrons.
Hence, carbonate of alkaline earths decomposes into oxide and CO2. Large cations like Ba2+ have low ionic potential and are unable to polarise the electrons of O atom due to which carbonates of large cations are more stable.
Safety
It may cause irritation. It is toxic. It should be handled carefully since several related beryllium compounds are known carcinogens.