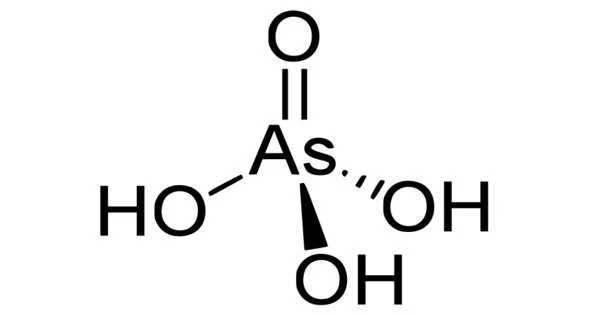
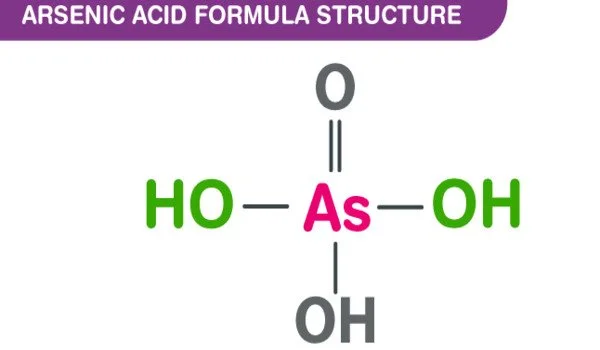
The chemical compound arsenic acid has the formula H3AsO4. It is an arsenic oxoacid with one hydroxy group and three oxo groups connected to a central arsenic atom. This colorless acid, also known as AsO(OH)3, is the arsenic counterpart of phosphoric acid. It is an arsenic oxoacid composed of three hydroxy and one oxo group. They are joined to the arsenic atom in the center. It is a metabolite of Escherichia coli.
Arsenate and phosphate salts have extremely comparable properties. Arsenic acid has not been isolated as such, but is only found in solution, where it is heavily ionized. It does form stable crystals in its hemihydrate form (H3AsO4• ½ H2O). At 100°C, crystalline samples dehydrate via condensation.
Properties
It is a colorless solution in aqueous form. It is white and translucent in its crystalline form. It is non-combustible and hygroscopic. It is a metabolite of Escherichia coli. It is a conjugate acid formed by an arsenate ion and an arsenate (1-).
- Chemical formula: H3AsO4
- Molar mass: 141.94 g/mol
- Appearance: White translucent crystals, hygroscopic.
- Density: 2.5 g/cm3
- Melting point: 35.5 °C (95.9 °F; 308.6 K)
- Boiling point: 120 °C (248 °F; 393 K) decomposes
- Solubility in water: 16.7 g/100 mL
- Solubility: soluble in alcohol
- Vapor pressure: 55 hPa (50 °C)
Being a triprotic acid, its acidity is described by three equilibria:
- H3AsO4 + H2O ⇌ H2AsO4– + H3O+ (pKa1 = 2.19)
- H2AsO4– + H2O ⇌ HAsO42- + H3O+ (pKa2 = 6.94)
- HAsO42- + H2O ⇌ AsO43- + H3O+ (pKa3 = 11.5)
These pKa values are close to those for phosphoric acid. The highly basic arsenate ion (AsO43-) is the product of the third ionization. Unlike phosphoric acid, arsenic acid is an oxidizer, as illustrated by its ability to convert iodide to iodine.
Preparation
Arsenic acid is prepared by treating arsenic trioxide with concentrated nitric acid. Dinitrogen trioxide is produced as a by-product.
As2O3 + 2 HNO3 + 2 H2O → 2 H3AsO4 + N2O3
The resulting solution is cooled to give colorless crystals of the hemihydrate H3AsO4· ½ H2O, although the dihydrate H3AsO4·2H2O is produced when crystallization occurs at lower temperatures.
Other methods
When arsenic pentoxide is dissolved in water and meta- or pyroarsenic acid is treated with cold water, arsenic acid is slowly produced. Arsenic acid can also be made directly from elemental arsenic by wetting it and treating it with ozone.
2 As + 3 H2O + 5 O3 → 2 H3AsO4 + 5 O2
Applications
Arsenic acid’s commercial applications are limited due to its toxicity. It is a precursor of a number of insecticides. It has been used as a wood preservative, a broad-spectrum biocide, a finishing agent for glass and metal, and a reagent in the manufacture of certain dyestuffs and organic arsenic compounds.
- It is occasionally used in wood preservatives.
- It is used as a reagent in some dyes.
- It is used as a finishing agent in metal and glass.
Safety Measures
- It is corrosive and highly toxic when ingested.
- It is a human carcinogen and a threat to the environment and therefore immediate steps should be followed to limit the spread.