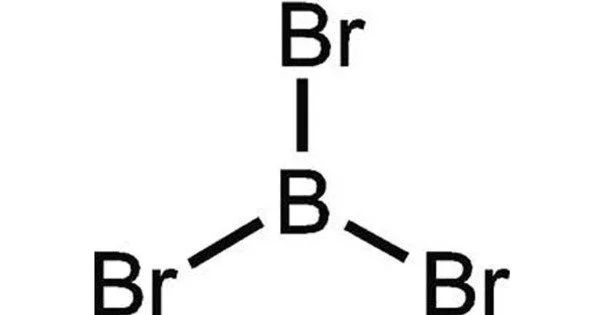
Boron tribromide (BBr3) is a colorless, fuming liquid compound made up of boron and bromine. Due to low bromine contamination, commercial samples typically range from amber to red/brown. Water and alcohols decompose it. It is widely used in pharmaceutical manufacturing, image processing, semiconductor doping, plasma etching, and photovoltaic manufacturing, as well as as a reagent in various chemical processes.
Properties
Boron tribromide appears as a colorless, fuming liquid with a pungent odor. Its boiling point is 194°F. Its freezing point is -51°F. It is very toxic by inhalation.
- Chemical formula: BBr3
- Molar mass: 250.52 g·mol−1
- Appearance: Colorless to amber liquid
- Odor: Sharp and irritating
- Density: 2.643 g/cm3
- Melting point: −46.3 °C (−51.3 °F; 226.8 K)
- Boiling point: 91.3 °C (196.3 °F; 364.4 K)
- Solubility in water: Reacts violently with water and other protic solvents
- Solubility: Soluble in CH2Cl2, CCl4
- Vapor pressure: 7.2 kPa (20 °C)
Chemical properties
Boron tribromide is a strong Lewis acid that is commercially available. It is an excellent demethylating or dealkylating agent for ether cleavage, with subsequent cyclization, which is frequently used in pharmaceutical production.
The mechanism of tertiary alkyl ether dealkylation involves the formation of a complex between the boron center and the ether oxygen, followed by the elimination of an alkyl bromide to yield a dibromo(organo)borane.
ROR + BBr3 → RO+(−BBr3)R → ROBBr2 + RBr
Aryl methyl ethers (as well as activated primary alkyl ethers), on the other hand are dealkylated through a bimolecular mechanism involving two BBr3-ether adducts.
RO+(−BBr)CH3 + RO+(−BBr3)CH3→ RO(−BBr3) + CH3Br + RO+(BBr2)CH3
The dibromo(organo)borane can then undergo hydrolysis to give a hydroxyl group, boric acid, and hydrogen bromide as products.
ROBBr2 + 3H2O → ROH + B(OH)3 + 2HBr
It is also used as a Lewis acid catalyst in olefin polymerization and Friedel-Crafts chemistry. Boron tribromide is used as a boron source in pre-deposition processes for semiconductor doping in the electronics industry. Boron tribromide also mediates aryl alkyl ether dealkylation, such as the demethylation of 3,4-dimethoxystyrene to 3,4-dihydroxystyrene.
History
The first synthesis was done by Poggiale in 1846 by reacting boron trioxide with carbon and bromine at high temperatures:
B2O3 + 3 C + 3 Br2 → 2 BBr3 + 3 CO
An improvement of this method was developed by F. Wöhler and Deville in 1857. By starting from amorphous boron the reaction temperatures are lower and no carbon monoxide is produced:
2 B + 3 Br2 → 2 BBr3
Synthesis
When boron carbide reacts with bromine at temperatures above 300 °C, boron tribromide is formed. Vacuum distillation can be used to purify the product.
Applications
Organic synthesis, pharmaceutical manufacturing, image processing, semiconductor doping, semiconductor plasma etching, and photovoltaic manufacturing all make use of boron tribromide.
Toxicity
Boron tribromide stimulates human tissue strongly, and its vapor is highly toxic and corrosive. During the operation, use masks, gloves, and protective clothing. Inhaling steam is strictly forbidden. After being poisoned, I was immediately taken to the hospital for treatment.