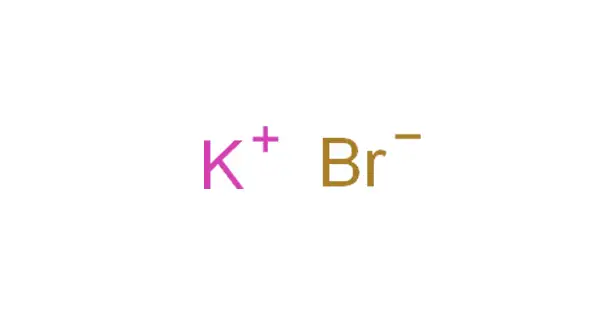Potassium bromide (KBr) is a salt that was widely used as an anticonvulsant and sedative in the late nineteenth and early twentieth centuries, with over-the-counter use in the United States lasting until 1975. The bromide ion is responsible for its action (sodium bromide is equally effective). Potassium bromide is a veterinary medicine that is used to treat epilepsy in dogs.
Potassium bromide is a white crystalline powder under normal circumstances. It is easily soluble in water but not in acetonitrile. Potassium bromide tastes pleasant in a weak aqueous solution, bitter at higher concentrations, and salty at even higher concentrations. These effects are primarily attributable to the characteristics of the potassium ion—at any concentration, sodium bromide tastes salty. Potassium bromide, in high concentrations, irritates the gastric mucous membrane, causing nausea and vomiting (a typical effect of all soluble potassium salts).
Properties
Potassium bromide appears as odorless colorless crystals or white crystalline powder or white granular solid with a pungent bitter saline taste.
- Chemical formula: KBr
- Molar mass: 119.002 g/mol
- Appearance: white solid
- Odor: odorless
- Density: 2.74 g/cm3
- Melting point: 734 °C (1,353 °F; 1,007 K)
- Boiling point: 1,435 °C (2,615 °F; 1,708 K)
- Solubility in water: 535 g/L (0 °C), 678 g/L (25 °C), 1020 g/L (100 °C)
- Solubility: very slightly soluble in diethyl ether

Chemical properties
Potassium bromide, a typical ionic salt, is fully dissociated and near pH 7 in aqueous solution. It serves as a source of bromide ions. This reaction is important for the manufacture of silver bromide for photographic film:
KBr(aq) + AgNO3(aq) → AgBr(s) + KNO3 (aq)
Aqueous bromide Br− also forms complexes when reacted with some metal halides such as copper(II) bromide:
2 KBr(aq) + CuBr2(aq) → K2[CuBr4](aq)
Preparation
A traditional method for the manufacture of KBr is the reaction of potassium carbonate with an iron(III, II) bromide, Fe3Br8, made by treating scrap iron under water with excess bromine:
4 K2CO3 + Fe3Br8 → 8 KBr + Fe3O4 + 4 CO2
Applications
Potassium bromide is used in veterinary medicine to treat epilepsy in dogs, either as first-line therapy or in combination to phenobarbital when phenobarbital alone does not control seizures. Bromide use in cats is restricted due to the significant danger of inducing lung inflammation (pneumonitis) in them. Because bromide is used as a therapeutic medicine for animals, veterinary medical diagnostic laboratories can routinely measure serum levels of bromide on the request of a veterinarian, however human medical diagnostic labs in the United States do not measure bromide as a routine test.
The US Food and Drug Administration (FDA) has not approved potassium bromide for use in humans to control seizures. It is still approved as an antiepileptic medicine for humans, particularly children and adolescents, in Germany. These indicators include severe kinds of generalized tonic-clonic seizures, tonic–clonic seizures in children, and severe myoclonic seizures in childhood. Adults who had a positive reaction to the medication as a kid or adolescent may continue treatment.
Uses
- Potassium Bromide is used to manufacture photographic papers and plates.
- Used as a laboratory agent.
- Used as heat stabilizer for nylon.
- Used as a Sedative.
- Used as an anticonvulsant.
- Used in the water treatment of aquariums
- Used to manufacture chemicals.
- Used as plasticizers.