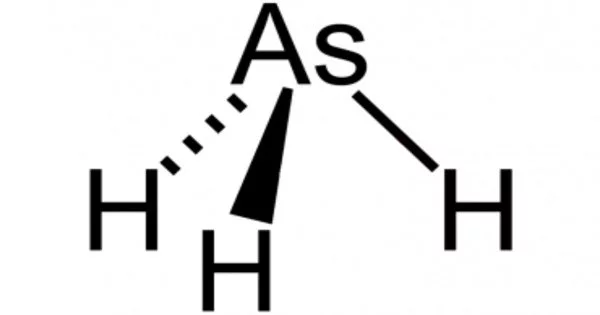
Arsine is an inorganic chemical having the formula AsH3. It is arsine, a member of the arsanes, and a mononuclear parent hydride. One of the most basic arsenic compounds is pnictogen hydride gas, which is volatile, pyrophoric, and very poisonous. Despite its toxicity, it has some applications in the semiconductor industry and in the creation of organoarsenic chemicals. It’s an arsonium conjugate base. It’s an arsanide conjugate acid.
The term arsine is commonly used to describe a class of organoarsenic compounds of the formula AsH3−xRx, where R = aryl or alkyl. For example, As(C6H5)3, called triphenylarsine, is referred to as “an arsine”.
General properties
Arsine is a colorless, flammable poisonous gas with a slight garlic odor. When arsenic reacts with an acid, arsine is generated. It is a denser-than-air gas that is mildly soluble in water (20% at 20 °C) and several organic solvents in its normal state. While arsine itself is odorless, due to air oxidation, it is possible to detect subtle garlic or fish-like odor when the component is present in concentrations greater than 0.5 ppm.
- Chemical formula: AsH3
- Molar mass: 77.9454 g/mol
- Appearance: Colourless gas
- Odor: unpleasant
- Density: 4.93 g/l, gas; 1.640 g/mL (−64 °C)
- Melting point: −111.2 °C (−168.2 °F; 162.0 K)
- Boiling point: −62.5 °C (−80.5 °F; 210.7 K)
- Solubility in water: 0.2 g/100 mL (20 °C); 0.07 g/100 mL (25 °C)
- Solubility: soluble in chloroform, benzene
- Vapor pressure: 14.9 atm
- Conjugate acid: Arsonium
This chemical is kinetically stable, meaning that it decomposes slowly at ambient temperature. Decomposition to arsenic and hydrogen occurs quickly enough at temperatures around 230 °C to serve as the basis for the Marsh Test. Arsine breakdown is autocatalytic, similar to stibine, because the arsenic liberated during the process acts as a catalyst for the same reaction. Several other elements, like as humidity, light, and particular catalysts (namely alumina), aid in the rate of disintegration.
Discovery and synthesis
AsH3 is generally prepared by the reaction of As3+ sources with H− equivalents.
4 AsCl3 + 3 NaBH4 → 4 AsH3 + 3 NaCl + 3 BCl3
As reported in 1775, Carl Scheele reduced arsenic(III) oxide with zinc in the presence of acid. This reaction is a prelude to the Marsh test, described below. Alternatively, sources of As3− react with protonic reagents to also produce this gas. Zinc arsenide and sodium arsenide are suitable precursors:
Zn3As2 + 6 H+ → 2 AsH3 + 3 Zn2+
Na3As + 3 HBr → AsH3 + 3 NaBr
Reactions
The understanding of the chemical properties of AsH3 is well developed and can be anticipated based on an average of the behavior of lighter pnictogen counterparts, such as PH3 and SbH3.
- Thermal decomposition
Typical for a heavy hydride (e.g., SbH3, H2Te, SnH4), AsH3 is unstable with respect to its elements. In other words, AsH3 is stable kinetically but not thermodynamically.
2 AsH3 → 3 H2 + 2 As
This decomposition reaction is the basis of the Marsh Test described below, which detects the elemental As.
- Oxidation
Continuing the analogy to SbH3, AsH3 is readily oxidized by concentrated O2 or the dilute O2 concentration in air:
2 AsH3 + 3 O2 → As2O3 + 3 H2O
Arsine will react violently in presence of strong oxidizing agents, such as potassium permanganate, sodium hypochlorite, or nitric acid.
Applications
This substance has varied uses.
It is used as a dopant in the semiconductor sector. Arsenic is an n-dopant in silicon and germanium when compared to P. More crucially, arsine is used in the chemical vapor deposition (CVD) of GaAs at temperatures ranging from 700 to 900 °C: Ga(CH3)3 + AsH3 GaAs + 3 CH4 GaAs + 3 CH4
Arsine gas was proposed for use in chemical warfare because it is colorless, practically odorless, and 2.5 times denser than air, making it suitable for a blanketing effect. Despite these advantages, arsine was never formally deployed as a weapon due to its extreme flammability and lower efficacy when compared to the non-flammable replacement phosgene.
It is sometimes used in soldering, galvanizing, burnishing, and lead plating.
Health Effects
Arsine is mostly absorbed through breathing. It is not bothersome and does not provide any immediate warning indications. Symptoms such as nausea, dizziness, and abdominal pain may occur within a few hours of coming into contact with 3 ppm arsine. Exposure to high levels of arsine may result in convulsions, paralysis, and respiratory failure.