Beryllium fluoride is employed in biochemistry, particularly protein crystallography, since it binds in human tissues in some of the same ways that phosphate does. ADP and beryllium fluoride tend to bind to ATP sites and block protein function, allowing proteins to crystallize in the bound state.
Beryllium fluoride is a fundamental component of the preferred fluoride salt mixture used in liquid-fluoride nuclear reactors. Typically, beryllium fluoride is combined with LiF to generate a base solvent into which uranium and thorium fluorides are added. Beryllium fluoride is chemically extremely stable, and LiF/BeF2 mixes have the lowest melting points and the highest neutronic characteristics of any fluoride salt combination suitable for reactor use.
Properties
It is slurry of white crystals. When dry, a high explosive that easily ignited and quick to burn vigorously. Wetting reduces sensitivity to shock and heat. It generates toxic oxides of nitrogen when burned. It is a colorless monoclinic crystal; density 2.936 g/cm3; decomposes at 120°C; soluble in water, slightly soluble in ethanol.
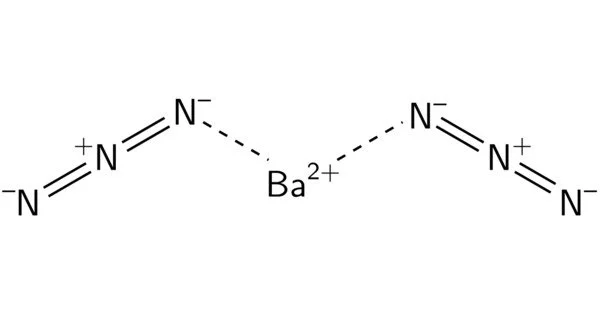
- Chemical formula: Ba(N3)2
- Molar mass: 221.37 g/mol
- Appearance: White crystalline solid
- Odor: Odourless
- Density: 2.936 g/cm3
- Melting point: 126 °C (259 °F; 399 K)
- Boiling point: 160 °C (320 °F; 433 K) (initial decomposition) > 225 °C explosion
- Solubility in water: 11.5 g/100 mL (0 °C); 15.36 g/100 mL (20 °C)
- Solubility in acetone: Insoluble
- Solubility in ether: Insoluble
Preparation
Barium azine can be made by reacting sodium azide with a soluble barium salt. Large crystals should be avoided in the solution since barium azide crystals burst when subjected to friction/shock or when fully dried. The product should be kept submerged in ethanol.
It is a suspension or slurry of an unstable solid in enough water to allow safe shipping and handling. If allowed to dry and then heated or jolted, it may explode. Incompatible with strong oxidizing and reducing agents.
Uses
Barium azide can be used to make azides of magnesium, sodium, potassium, lithium, rubidium and zinc with their respective sulfates.
Ba(N3)2 + Li2SO4 → 2 LiN3 + BaSO4
It can also be used as a source for high pure nitrogen by heating:
Ba(N3)2 → Ba + 3 N2
This reaction liberates metallic barium, which is used as a getter in vacuum applications.
Health Hazard
Some are toxic and, if inhaled, eaten, or absorbed via the skin, can be lethal. Skin and eye burns may result from contact. Fire can emit irritant, corrosive, and/or poisonous gases. Pollution may result from runoff from firefighting or diluting water.