Atoms and molecules, the fundamental constituents of matter, interact with one another. They are attracted over long distances, but interactions are strongly repulsive over short distances. In fact, there is no need to seek evidence that such interactions exist. We see the indirect effects of these interactions with our own eyes every day. Water molecules, for example, stick together to form drops, which then rain down to fill rivers due to their attraction. However, due to the strong repulsion, one liter of the same water always weighs about 1 kg, no matter how much pressure we apply to compress it.
Researchers used a clever combination of scanning transmission electron microscopy and video-based tracking to observe and characterize dynamic assembly of metallic atoms. The researchers open up the possibility of observing more such dynamic structures predicted by simulations by visualizing short-lived molecules, such as metallic dimers and trimers, that cannot be observed using traditional methods.
Chemistry is the study of atom bond formation (or dissociation). Understanding how chemical bonds form is fundamental to not only all of chemistry, but also to fields such as materials science. Traditional chemistry, on the other hand, has been largely limited to the study of stable compounds. Little attention has been paid to the study of dynamic assembly of atoms during a chemical reaction.
Real-time tracking of the moving atoms enabled elemental identification, while ADF-STEM allowed the atoms to be observed under electron dose. This allowed us to avoid the high current densities required for single-atom analysis, which can cause material damage.
Dr. Takane Imaoka
However, with recent advances in computational chemistry, dynamic, short-lived structures are becoming more important. The experimental observation and characterization of predicted dynamic bonding between atoms, such as the formation of metallic dimers, could open up new research avenues in chemistry and materials science.
However, observing this bond dynamics also requires the development of a new methodology. This is because conventional characterization techniques only provide a time-averaged structural information and are, thus, inadequate for observing the bonds as they are formed.
In light of this, Japanese researchers led by Associate Professor Takane Imaoka of Tokyo Institute of Technology (Tokyo Tech) have devised an ingenious solution. The team used video tracking and a technique called “annular dark field scanning transmission electron microscopy” (ADF-STEM) to perform sequential imaging of different metal atoms interacting with one another in their study published in Nature Communications. This enabled them to directly observe transient structures formed by the assembly of two similar atoms (homo-metallic dimers), two different atoms (hetero-metallic dimers), and three different atoms (hetero-metallic dimers) (hetero-metallic trimers).
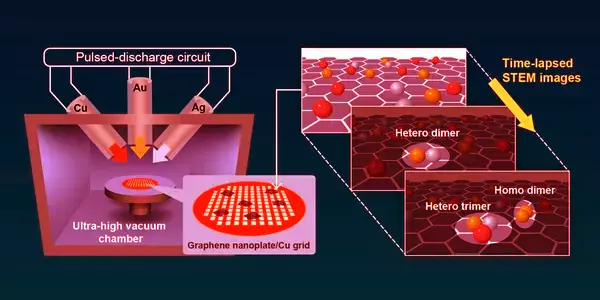
The researchers began by depositing atoms of gold (Ag), silver (Ag), and copper (Cu) on a graphene nanoplate using a technique known as “arc-plasma deposition.” To ensure that there were enough isolated single atoms available, the deposition was limited to 0.05-0.015 monolayers, and high-magnification observations were performed on the flat regions of the graphene substrate.
“Real-time tracking of the moving atoms enabled elemental identification, while ADF-STEM allowed the atoms to be observed under electron dose. This allowed us to avoid the high current densities required for single-atom analysis, which can cause material damage” Dr. Imaoka explains.
ADF-STEM imaging also demonstrated extremely high atom discrimination accuracy, ranging from 98.7 percent for Au-Ag pairs to 99.9 percent for Au-Cu pairs. Other pairings demonstrated comparable levels of discrimination. Furthermore, the team observed Au-Ag-Cu, an extremely short-lived heterometallic trimer.
“Although our snapshots did not perfectly agree with the structures predicted by theoretical calculations,” Dr. Imaoka says, “the average bond lengths between the elements in the observed structures are in good agreement with the calculations.”
The remarkable findings of this study could lead to rapid advances in nanoscience, where the characterization of metal clusters and subnanoparticles is gaining importance, and, in the process, open doors to a completely new realm of matter.