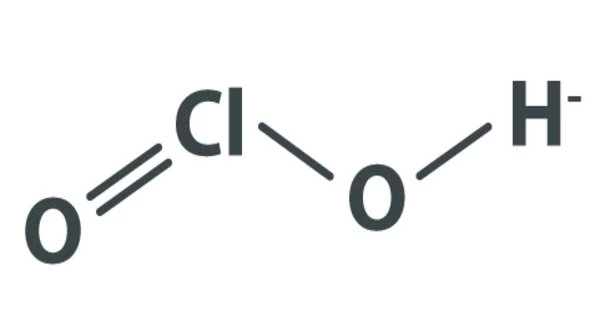
Chlorous acid has the formula HClO2 and is an inorganic compound. It’s a mild acid. In this acid, chlorine has an oxidation state of +3. Because the acid is unstable, it cannot be isolated in its pure form. Chlorous acid is a chlorine oxoacid derived from chlorine dioxide (ClO2), a strong oxidizer. The pure substance is unstable, yielding hypochlorous acid (Cl oxidation state +1) and chloric acid (Cl oxidation state +5):
2 HClO2 → HClO + HClO3
Although the acid is difficult to obtain in pure form, the conjugate base derived from it, chlorite, is stable. The well-known sodium chlorite is an example of an anion salt. This and related salts are occasionally used in the manufacture of chlorine dioxide.
Properties
- Molecular Weight: approximately 68.46 g/mol.
- Structure: central chlorine atom bonded to two oxygen atoms and one hydrogen atom [H-O-Cl=O]
- Stability: thermally unstable and readily decomposes into chlorine dioxide (ClO2) and water (H2O) through a disproportionation reaction
- Oxidizing Agent: it can accept electrons from other substances during chemical reactions
- Solubility: It is soluble in water and forms an acidic solution
Preparation
Chlorous acid can be formed by dissolving chlorine dioxide gas in water or by the reaction of chlorine dioxide with a strong acid. It primarily exists as its salts, known as chlorites, which are more stable than the acid. Common chlorites include sodium chlorite (NaClO2) and potassium chlorite (KClO2).
HClO2 can be prepared through reaction of barium or lead chlorite and dilute sulfuric acid:
Ba(ClO2)2 + H2SO4 → BaSO4 + 2 HClO2
Pb(ClO2)2 + H2SO4 → PbSO4 + 2 HClO2
Stability
Chlorous acid is a powerful oxidizing agent, but its disproportionation tendency negates its oxidizing potential. Chlorine is the only halogen that can form the isolable acid HXO2. Bromous acid and iodous acid have never been isolated. There are a few bromites, salts of bromous acid, but no iodites.
Application
Chloric acid and its salts are used in a variety of industries. Sodium chlorite, for example, is used as a disinfectant, bleaching agent, and in water treatment processes. It has the ability to produce chlorine dioxide, which has potent antimicrobial properties. Chlorous acid and chlorites are also used to make dyes, paper, and textiles.