Ammonium azide is the salt of ammonia and hydrazoic acid and has the chemical formula [NH4]N3. It is a very explosive and highly sensitive substance that must be handled with utmost caution. This colorless crystalline salt, like other inorganic azides, is a strong explosive with a relatively low sensitivity. It is rarely met in regular life, and its primary application is in laboratory research or specialized applications.
[NH4]N3 is physiologically active, causing headaches and palpitations when inhaled in modest doses. Theodor Curtius discovered it, along with other azides, in 1890. It can quickly detonate due to its great sensitivity to heat, shock, or friction, making it risky to handle without sufficient training and safety precautions.
Properties
- Chemical formula: [NH4]N3
- Molar mass: 60.059 g/mol
- Appearance: White crystalline solid
- Odor: Odorless
- Density: 1.3459 g/cm3
- Melting point: 160 °C (320 °F; 433 K)
- Boiling point: 400 °C (752 °F; 673 K) (decomposes)
- Crystal structure: Orthorhombic
Structure
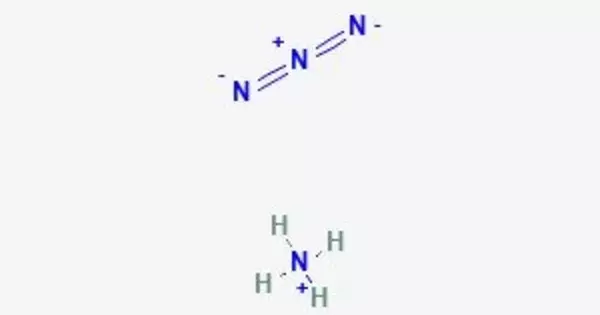
Ammonium azide is ionic, meaning it consists of ammonium cation [NH4]+ and azide anion N−3, therefore its formula is [NH4]+[N3]−. It is a structural isomer of tetrazene. Ammonium azide contains about 93% nitrogen by mass.
Decomposition
Ammonium azide is a white, crystalline powder or solid. It is typically odorless. It is highly sensitive to shock, heat, and friction. Even slight disturbances can lead to its rapid decomposition and release of toxic and explosive nitrogen gas. It should be handled with great caution to avoid accidents. When subjected to heat or mechanical shock, ammonium azide readily decomposes into nitrogen gas (N2) and ammonia gas (NH3). This reaction is highly exothermic and can result in an explosion.
Stability
Ammonium azide is not stable and should be stored under controlled conditions to prevent its decomposition. It should be kept away from heat, open flames, and incompatible materials. The decomposition products of ammonium azide, such as ammonia, can be toxic if inhaled. In addition to the explosion risk, exposure to these gases can pose serious health hazards.
Uses
Ammonium azide has few practical applications due to its high sensitivity and explosive qualities. Its fascinating chemistry and utility as a precursor for the synthesis of additional azides and nitrogen-containing compounds make it popular in academic research.
Safety
Because ammonium azide is explosive, it must be handled and stored with extreme caution and in accordance with rigorous safety regulations. Ammonium azide should be avoided in direct contact because it can cause severe skin and eye irritation. Inhaling its vapors can cause respiratory problems. Immediate medical assistance is required in the event of exposure or ingestion.