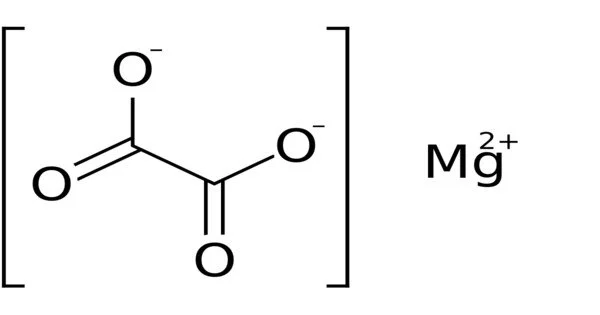
Magnesium oxalate is a salt that is formed by the combination of magnesium and oxalic acid. It can be found naturally in many plants and is also present in some kidney stones. Magnesium oxalate is not very soluble in water and has a low solubility product, which means that it does not easily dissociate into its ions in solution. It can be used as a reagent in laboratory experiments, and in some industrial applications such as textile and paper manufacturing. High levels of oxalate in the urine is a risk factor for the formation of kidney stones, thus excessive intake of food high in oxalate should be avoided for those at risk of this condition.
it is a strong dicarboxylic acid occurring in many plants and vegetables. It is produced in the body by metabolism of glyoxylic acid or ascorbic acid. It is not metabolized but excreted in the urine. It is used as an analytical reagent and general reducing agent.
Properties
Magnesium oxalate is an organic compound composed of a 2+ charged magnesium cation bonded to an oxalate anion. Its chemical formula is MgC2O4. Magnesium oxalate is a white solid that exists in two forms: anhydrous and dihydrate, the latter of which contains two water molecules complexed with the structure. Both types are practically insoluble in water and inorganic solutions.
- Chemical formula: MgC2O4; [MgC2O4•2H2O (Dihydrate)]
- Molar mass: 112.324 g/mol; 148.354 g/mol (Dihydrate)
- Appearance: white solid
- Density: 2.45 g/cm3
- Melting point between: 420 and 620 °C (788 and 1,148 °F; 693 and 893 K); 150 °C (302 °F; 423 K) (dihydrate) both decompose
- Boiling point: Not Applicable
- Solubility in water: 0.038g/100g H2O (anhydrous and dihydrate)

Natural occurrence
Magnesium oxalate has been discovered naturally near Mill of Johnston, near Insch in northeast Scotland. This naturally occurring magnesium oxalate is known as glushinskite, and it appears as a creamy white layer mixed in with the hyphae of the lichen fungus at the lichen/rock interface on serpentinite. A scanning electron micrograph of the samples revealed a pyramidal structure with both curved and striated faces. The size of these crystals ranged from 2 to 5 μm.
Health and safety
Magnesium oxalate irritates the skin and the eyes. It will irritate the lungs and mucous membranes if inhaled. Magnesium oxalate has no known long-term or carcinogenic effects. Magnesium oxalate is non-flammable and stable, but it emits toxic fumes in a fire. According to OSHA, magnesium oxalate is a hazardous substance.