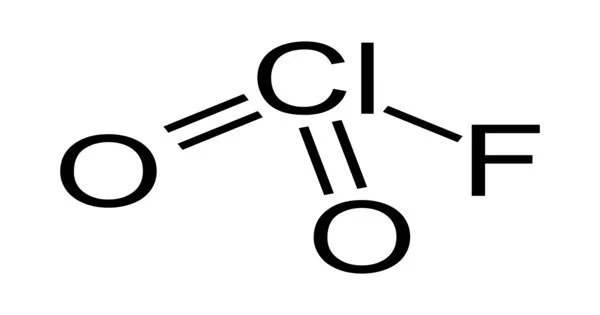
Chloryl fluoride is a chemical compound with the formula ClO2F. It is a common byproduct of chlorine fluoride reactions with oxygen sources. It is the chloric acid acyl fluoride. The chemical formula for chloryl fluoride shown above is based on the molecular formula, which indicates the numbers of each type of atom in a molecule without structural information, as opposed to the empirical formula, which provides the numerical proportions of atoms of each type.
The chemical structure of a molecule includes the arrangement of atoms as well as the chemical bonds that hold the atoms together. There are three bonds in the chloryl fluoride molecule (s) There are 3 non-H bond(s), 2 multiple bond(s), and 2 double bond(s) (s).
Properties
- Chemical formula: ClFO2
- Molar mass: 86.45 g·mol−1
- Density: 3.534 g/L
- Melting point: −115 °C
- Boiling point: −6 °C
- Related compounds: Perchloryl fluoride
Preparation
Schmitz and Schumacher reported ClO2F for the first time in 1942, after fluorinating ClO2. The compound is more easily prepared by treating sodium chlorate and chlorine trifluoride and purifying it by vacuum fractionation, which involves selectively condensing this species from other products. This species is a gas that boils at -6 °C:
6 NaClO3 + 4 ClF3 → 6 ClO2F + 2 Cl2 + 3 O2 + 6 NaF
SO2ClF (sulfuryl chloride fluoride) is a strong Lewis acid that can be used as a solvent and reagent. It must be handled with caution, as shown in the hazard information table. It has a boiling point that allows it to be used as a gas or a liquid.
Structure
ClO2F is a pyramidal molecule, as opposed to O2F2. VSEPR predicts this structure. The differences in structure reflect chlorine’s greater proclivity to exist in positive oxidation states with oxygen and fluorine ligands. Perchloryl fluoride, ClO3F, is a tetrahedral Cl-O-F compound. Bromyl fluoride (BrO2F), a related bromine compound, has the same structure as ClO2F, whereas iodyl fluoride (IO2F) forms a polymeric substance under standard conditions.