Trihydrogen oxide has the chemical formula H3O and is a hypothesized inorganic compound of hydrogen and oxygen. One of the unstable hydrogen polyoxides, this is still a speculative substance. It is one of the most plentiful and necessary elements on Earth, accounting for a large amount of the planet’s surface as well as the bulk of living organisms. Water is a polar molecule made up of two hydrogen atoms that are linked to one oxygen atom.
The chemical is expected to form a thin layer of metallic liquid around the cores of Uranus and Neptune, serving as the source of their magnetic fields. Calculations show that H3O is stable in solid, superionic, and fluid metallic forms in these planets’ deep interiors.
Synthesis
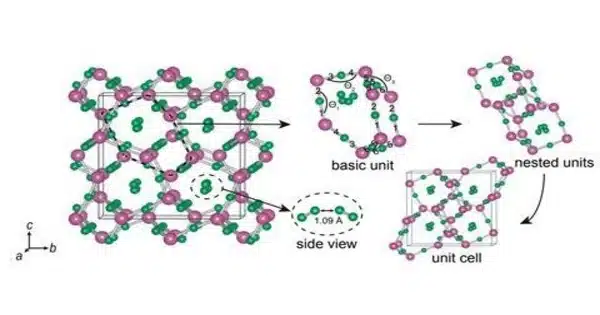
Trihydrogen oxide has not been observed experimentally as of 2023, but its existence is predicted by calculation using the CALYPSO method. The compound should be stable in the pressure range 450–600 GPa and could be produced by the reaction:
2H2O + H2 → 2H3O
Physical properties
The compound is considered not a true molecular trihydrogen oxide compound. Instead, each oxygen atom is linked by a strong (covalent) bond to only two hydrogen atoms, as a water molecule, and there are molecules of dihydrogen inserted in the voids of the water molecules network. Structurally, it is thus a 2(H2O)·H2 stoichiometric combination.
- Density: The density varies with temperature. At 25°C (77°F), the density is approximately 0.997 grams per cubic centimeter (g/cm³). Water’s density decreases as it freezes, which is why ice floats on liquid water.
- Melting Point: It freezes at 0°C (32°F) under normal atmospheric pressure.
- Boiling Point: It boils at 100°C (212°F) under normal atmospheric pressure.
- Heat Capacity: It has a relatively high specific heat capacity, meaning it can absorb and store a significant amount of heat energy without a large increase in temperature. This property helps regulate Earth’s climate and temperature.
Solvent Properties
It is known as the “universal solvent” because it can dissolve a large variety of compounds, making it necessary for a variety of chemical reactions and biological activities. Surface tension occurs as a result of the cohesive forces between water molecules at the surface. Small items can float on the surface of water due to this feature.
It is a polar molecule, which means that the hydrogen atoms have a partial positive charge while the oxygen atom has a partial negative charge. This polarity produces features such as hydrogen bonding, which is vital in a variety of biological and chemical processes.
It has a high heat of vaporization, which means it takes a lot of energy to transform from a liquid to a gas. This feature is essential for cooling systems in living creatures and aids in the regulation of Earth’s temperature.
It is required for life on Earth and is an important component of biological processes. It acts as a medium for chemical reactions, transports nutrients and waste, and aids in the maintenance of temperature stability in living organisms.