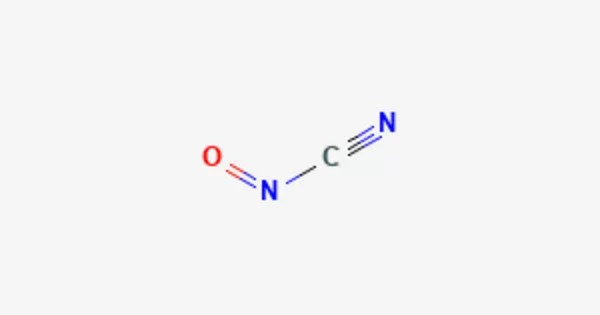
Nitrosyl cyanide is a blue-green gas having the chemical formula ONCN. It’s a chemical compound made up of nitrosyl (NO) and cyanide (CN) groups. The chemical has been identified as a byproduct of the cyanamide oxidation mediated by the enzyme glucose oxidase. It is a highly poisonous, potentially explosive molecule that has mostly been explored for its role in coordination chemistry and prospective uses in materials science.
Preparation
Nitrosyl cyanide can be made through a variety of chemical reactions that involve the mixing of multiple chemicals, although it is not a frequent laboratory reagent because to its instability and toxicity. It is a highly reactive chemical that is unstable under normal circumstances. It decomposes quickly and emits toxic fumes, making it a dangerous chemical to handle.
Structure, synthesis, reactivity
Nitrosyl cyanide has a planar structure. It has a sharply bent internal nitrogen, similar to the structure of nitrosyl chloride. The C-N-O angle is 113 degrees. The NCN angle is 170 degrees. A nitrosyl group (NO) is attached to a cyanide group (CN) to form the molecule. The nitrosyl group’s nitrogen atom is connected to the cyanide group’s carbon atom. The structure is represented by the letters NO-CN.
Low-temperature reactions of nitrosyl chloride and silver cyanide can produce the chemical. It is usually trapped by Diels-Alder reactions, such as with butadiene. Cycloadditions take place across the N=O bond. When combined with 9,10-dimethylantracene, it creates a reversible adduct.
Properties
- Chemical formula: CN2O
- Molar mass: 56.024 g·mol−1
- Appearance: blue-green gas
Coordination Chemistry
It is of interest in coordination chemistry because it can form coordination complexes with transition metals. These complexes have been studied for their electronic and magnetic properties.
Applications
While nitrosyl cyanide has few practical applications due to its toxicity and instability, its study in coordination chemistry contributes to our understanding of chemical bonding and metal complex behavior. These discoveries could have far-reaching ramifications in the development of novel materials and catalysts.
Toxicity
Nitrosyl cyanide is highly poisonous and should be handled with extreme caution. Exposure to this substance can result in serious health hazards such as respiratory, cardiovascular, and neurological issues. It is explosive under specific conditions due to its sensitivity to shock, heat, and friction. As a result, it is mostly employed as a research substance rather than an explosive.