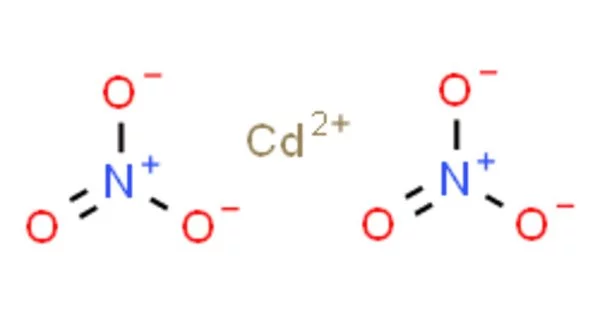
Cadmium Nitrate is a colorless, crystalline inorganic compound that, when heated, emits toxic cadmium oxide fumes. It refers to any of the related members of an inorganic compound family, with the tetrahydrate being the most commonly encountered form. The anhydrous form is volatile, but the others are colorless crystalline solids that are deliquescent, absorbing enough moisture from the air to form an aqueous solution. Cadmium nitrate, like other cadmium compounds, is carcinogenic.
Calcium nitrate is used in the production of cadmium hydroxide, which is used in alkaline batteries, to color glass and porcelain, in photography, and in nuclear reactors.
Properties
- Chemical formula: Cd(NO3)2
- Molar mass: 236.42 g/mol (anhydrous); 308.48 g/mol (tetrahydrate)
- Appearance: White crystals, hygroscopic
- Odor: Odorless
- Density: 3.6 g/cm3 (anhydrous); 2.45 g/cm3 (tetrahdyrate)
- Melting point: 360 °C (680 °F; 633 K)
- Boiling point: 132 °C (270 °F; 405 K)
- Solubility in water: 109.7 g/100 mL (0 °C); 139.8 g/100 mL (30 °C)
- Solubility: Soluble in acids, ammonia, alcohols, ether, acetone
- Crystal structure: Cubic (anhydrous); Orthorhombic (tetrahydrate)
Cadmium was discovered and isolated for the first time in 1817 by Karl Samuel Leberecht Hermann and Friedrich Stromeyer. It has a silvery bluish-gray metallic appearance in its elemental form. Cadmium nitrate is also known as Cadmium (II) nitrate or Cadmium dinitrate. It is a colorless inorganic crystalline compound. When heated, it emits toxic cadmium oxide fumes.

Preparation
Cadmium nitrate is prepared by dissolving cadmium metal or its oxide, hydroxide, or carbonate, in nitric acid followed by crystallization:
CdO + 2HNO3 → Cd(NO3)2 + H2O
CdCO3 + 2 HNO3 → Cd(NO3)2 + CO2 + H2O
Cd + 4 HNO3 → 2 NO2 + 2 H2O + Cd(NO3)2
Reactions
Thermal dissociation at high temperatures results in cadmium oxide and nitrogen oxides. Yellow cadmium sulfide is formed when hydrogen sulfide is passed through an acidified solution of cadmium nitrate. Under boiling conditions, a red modification of the sulfide is formed.
Cadmium oxide precipitates as cadmium hydroxide in caustic soda solution. Precipitation reactions produce a large number of insoluble cadmium salts.
Uses
Cadmium nitrate is used to color glass and porcelain, as well as in photography as a flash powder. It is commonly used in the manufacture of cadmium hydroxide. It is used to tint porcelain and glass.
Safety hazards
This substance irritates the eyes, skin, and respiratory tract, damages the lungs, causing shortness of breath, chest pain, and pulmonary edema, and can also damage the kidneys, causing proteinuria and decreased renal function. It is a known carcinogen and has been linked to an increased risk of developing lung cancer.