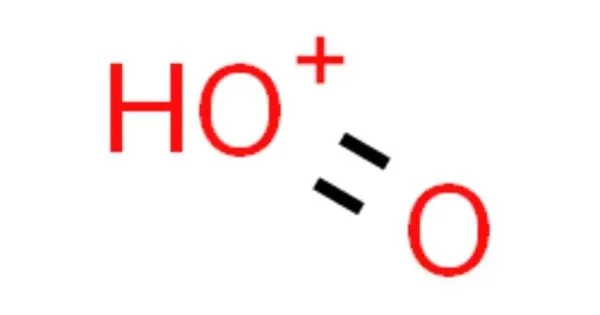
The ion dioxidanylium, also known as protonated molecular oxygen or simply protonated oxygen, has the formula HO+2. When water (H2O) gains a proton (H+), it forms hydronium (H3O+). It is produced when hydrogen-containing substances combust and can be found in the ionosphere and plasmas containing oxygen and hydrogen. The production of protonated molecular oxygen may occur during O2 oxidation in superacids.
It is the dioxygen conjugate acid. Dioxygen (O2) has a proton affinity of 4.4 eV. It is made up of three hydrogen atoms that are bonded to an oxygen atom. Because of the extra proton, the central oxygen atom has a positive charge. The molecule has a trigonal pyramidal geometry, with the oxygen atom at the apex and the three hydrogen atoms forming the base.
Properties
Dioxidanylium is a strong acid that is commonly used as an example of a Brnsted acid. Because it readily donates the proton it carries, it is an important component in acid-base reactions. It is commonly found in acidic aqueous solutions. The v1 band in the HO+2 infrared spectrum has a band head at 3016.73 cm-1 due to vibrating O-H.
- Chemical formula: HO2+
- Molar mass: 33.005 g·mol−1
Water molecules interact with dioxidanylium via hydrogen bonding in aqueous solutions. The dioxidanylium ion’s oxygen atom forms hydrogen bonds with nearby water molecules, yielding the hydronium ion (H3O+). This procedure is critical for comprehending the behavior of acids in solution.
Significance
In order to detect dioxygen in space, protonated molecular oxygen is of interest. Because the Earth’s atmosphere is dense with O2, observing the spectrum of a space object from the ground is impossible. HO+ 2 should, however, be much more detectable.
Because of the positive charge on the oxygen atom, dioxidanylium is a highly reactive species. It is involved in a variety of chemical reactions, particularly acid-base reactions. When it reacts with bases, it produces water and a salt. It can also react with nucleophiles like hydroxide ions to form water and the corresponding nucleophile.
Reactions
A helium complex (He–O2H+) also is known.
HO+2 appears to react rapidly with hydrogen:
HO+2 + H2 → O2 + H+3
HO+2 also reacts with dinitrogen and water:
HO+2 + H2O → O2 + H3O+
As the primary reactive species in acid-base chemistry, dioxidanylium plays an important role in water. It allows for the formation and dissociation of various ions by facilitating the transfer of protons between acids and bases. The concentration of dioxidanylium ions in a solution determines its pH, with higher concentrations resulting in more acidic conditions.