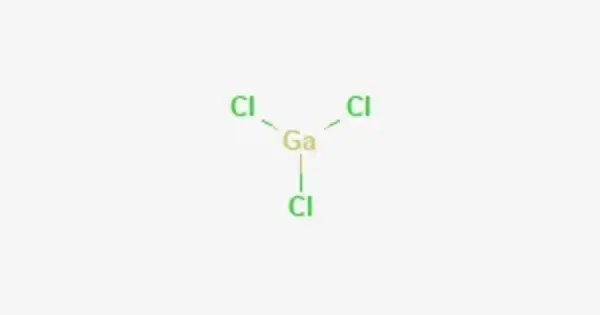
Gallium trichloride is a chemical compound with the formula GaCl3. It is a chemical compound composed of gallium and chlorine atoms. Solid gallium trichloride exists as a dimer with the formula Ga2Cl6. It is colorless and soluble in virtually all solvents, even alkanes, which is truly unusual for a metal halide. It is the main precursor to most derivatives of gallium and a reagent in organic synthesis.
Gallium trichloride is highly reactive and can react violently with water, releasing hydrogen chloride gas. It also reacts with strong bases and oxidizing agents. It is highly reactive and is primarily used in chemical vapor deposition (CVD) processes for the production of thin films and coatings. It is also employed as a catalyst in various organic synthesis reactions.
Properties
- Physical state: It is typically found as a colorless to pale yellow solid at room temperature.
- Melting Point: It melts at around 77.9°C (172.22°F).
- Boiling Point: It has a boiling point of approximately 201°C (394°F).
- Solubility: It is soluble in common organic solvents like benzene and chloroform. However, it is insoluble in water.
- Odor: It has a pungent odor.
- Structure: In the solid state, it adopts a layered structure.
Preparation
Gallium trichloride can be prepared from the elements, heating gallium metal in a stream of chlorine, and purifying the product by sublimation under vacuum.
2 Ga + 3 Cl2 → 2 GaCl3
It can also be prepared from by heating gallium oxide with thionyl chloride:
Ga2O3 + 3 SOCl2 → 2 GaCl3 + 3 SO2
Gallium metal reacts slowly with hydrochloric acid. This reaction produces hydrogen gas slowly.
Applications
Gallium trichloride has applications in chemical synthesis, particularly in the production of gallium metal. It is also used as a catalyst in organic synthesis, especially in Friedel-Crafts reactions. Additionally, it has been studied for potential use in the fabrication of thin films and semiconductors.
Toxicity
Like many metal chlorides, gallium trichloride can be toxic and should be handled with care. It can cause irritation to the skin, eyes, and respiratory system upon contact or inhalation.