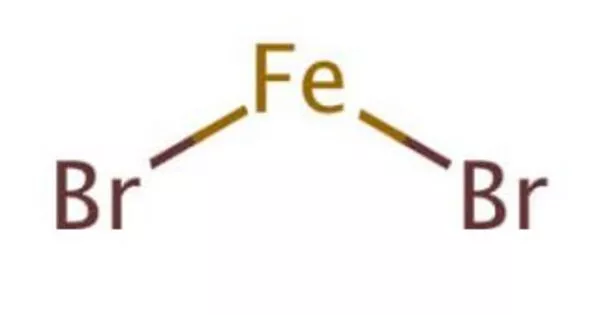
Iron(II) bromide is a chemical compound with the formula FeBr2. The anhydrous compound is a paramagnetic solid that is yellow or brownish in color. Several FeBr2 hydrates are also known, all of which are pale-colored solids. It is a common precursor to other iron compounds in research laboratories, but it has no applications.
Iron(II) bromide can be made by reacting bromine or hydrogen bromide directly with iron at high temperatures. It is a catalyst for polymerization. It is used in water treatment, chemical analysis, and ultra-pure crystal growth applications.
Properties
The chemical compound iron(II) bromide This brownish-colored solid is a useful synthetic intermediate that is used to insert Fe(II) into porphyrins, for example. Bromine is a halogen element with the atomic number 35 and the symbol Br. Although diatomic bromine does not exist in nature, bromine salts can be found in crustal rock.
- Chemical formula: FeBr2
- Molar mass: 215.65 g mol−1
- Appearance: yellow-brown solid
- Density: 4.63 g cm−3, solid
- Melting point: 684 °C (1,263 °F; 957 K) (anhydrous); 27 °C (Hexahydrate)
- Boiling point: 934 °C (1,713 °F; 1,207 K)
- Solubility in water: 117 g / 100 ml
- Solubility in other solvents: THF, methanol, ethanol
Structure
FeBr2, like most metal halides, has a polymeric structure made up of isolated metal centers that are cross-linked with halides. It crystallizes with the CdI2 structure, which consists of close-packed layers of bromide ions separated by octahedral holes filled with Fe(II) ions. The halide packing differs slightly from that of FeCl2, which uses the CdCl2 motif. FeX2(H2O)4 (X = Cl, Br) tetrahydrates have similar structures, with octahedral metal centers and mutually trans halides.
Synthesis and reactions
FeBr2 is synthesized using a methanol solution of concentrated hydrobromic acid and iron powder. It adds the methanol solvate [Fe(MeOH)6]Br2 together with hydrogen gas. Heating the methanol complex in a vacuum gives pure FeBr2.
FeBr2 reacts with two equivalents of tetraethylammonium bromide to give [(C2H5)4N]2FeBr4. FeBr2 reacts with bromide and bromine to form the intensely colored, mixed-valence species [FeBr3Br9]–.
Magnetism
FeBr2 possesses a strong metamagnetism at 4.2 K and has long been studied as a prototypical metamagnetic compound.