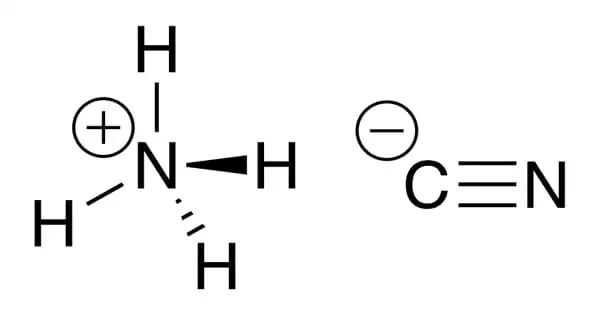
Ammonium cyanide has the formula NH4CN and is an unstable inorganic chemical. A cyanide is a chemical compound that has the CN group in its name. A carbon atom is triple-bonded to a nitrogen atom to form the cyano group. Due to its instability, it is not exported or sold commercially. Ammonium cyanide is extensively used in organic synthesis. Because of its instability, it cannot be exported or sold commercially.
Properties
- Melting point: 36°C
- Boiling point: 31.7°C (estimate)
- Density: 1.100
- Form: colorless tetragonal crystal
Preparation
Ammonium cyanide is prepared in solution by bubbling hydrogen cyanide into aqueous ammonia at a low temperature
HCN + NH3 (aq) → NH4CN (aq)
It may be prepared by the reaction of calcium cyanide and ammonium carbonate:
Ca(CN)2 + (NH4)2CO3 → 2 NH4CN + CaCO3
In dry state, ammonium cyanide is made by heating a mixture of potassium cyanide or potassium ferrocyanide with ammonium chloride and condensing the vapours into ammonium cyanide crystals:
KCN + NH4Cl → NH4CN + KCl
Reactions
Ammonium cyanide decomposes to ammonia and hydrogen cyanide, often forming a black polymer of hydrogen cyanide:
NH4CN → NH3 + HCN
It undergoes salt metathesis reaction in solution with a number of metal salts to form metal–cyanide complexes.
Reaction with ketones and aldehydes yield aminonitriles, as in the first step of the Strecker amino acid synthesis:
NH4CN + CH3COCH3 → (CH3)2C(NH2)CN + H2O
Uses
Ammonium cyanide is generally used in organic synthesis. Being unstable, it is not shipped or sold commercially.
Cyanide is mostly used in gold and silver mining because it aids in the dissolution of these metals and their ores. Finely ground high-grade ore is combined with cyanide in the cyanide process; low-grade ores are stacked into heaps and sprayed with a cyanide solution in the cyanide process.
Toxicity
The solid or its solution is highly toxic. Ingestion can cause death. Exposure to the solid can be harmful as it decomposes to highly toxic hydrogen cyanide and ammonia.