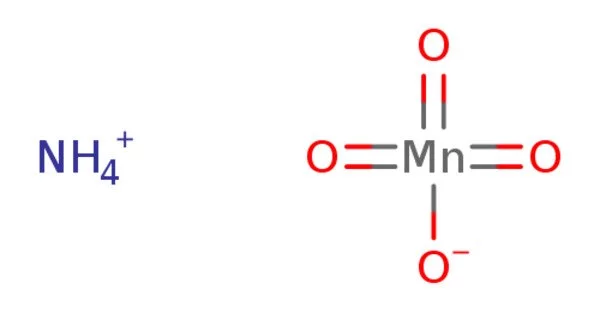
The chemical formula for ammonium permanganate is NH4 MnO4. It is a highly active chemical compound. It is both a strong oxidizer and a moderately strong explosive. It is a violet-brown or dark purple salt that is water soluble. It decomposes rapidly, emitting nitrogen, water vapors, and a dense cloud of dark brownish manganese dioxide dust.
Properties
Decomposition of ammonium permanganate is explosive, releasing nitrogen, water vapors, and a dense cloud of dark brownish manganese dioxide dust. It is a violet-brown or dark purple metallic crystalline or powder that eventually turns steel-gray. The color of aqueous solutions is magenta-rose. It is only slightly soluble in water and completely insoluble in acetone.
- Chemical formula: NH4MnO4
- Molar mass: 136.974 g/mol
- Appearance: rhombic needle crystals or powder with rich violet-brown or dark purple metallic sheen, become steel-gray in storage; magenta–rose in solution
- Density: 2.2g/cm3, solid
- Melting point: decomposes
- Solubility in water: 8.0 g/100 ml at 15 °C
- Crystal structure: Orthorhombic
Preparation
Eilhard Mitscherlich invented ammonium permanganate in 1824 by reacting silver permanganate with an equal molar amount of ammonium chloride, filtering the silver chloride, and evaporating the water.
Ammonium permanganate can be made in a variety of ways. The best involves the reaction of silver permanganate and ammonium chloride in water. Silver chloride precipitates and is removed through filtration. The aqueous ammonium permanganate solution is concentrated by vacuum evaporation and then cooled to precipitate NH4MnO4 crystals. Potassium permanganate can also be used.
AgMnO4 + NH4Cl → AgCl + NH4MnO4
It can also be prepared in a similar way from barium permanganate and ammonium sulfate.
Ba(MnO4)2 + (NH4)2SO4 → BaSO4 + 2 NH4MnO4
Explosive
Because of the combination of the oxidizer permanganate anion and the reducing ammonium cation, ammonium permanganate is a moderately strong explosive. Dry ammonium permanganate is heat, shock, and friction sensitive, and it can explode at temperatures above 60 °C (110 °C according to Crespi). The detonation of this compound is not particularly violent, and can be accomplished with an open flame, which emits copious amounts of dark brown powder smoke while also projecting leftover material in all directions. It has no oxygen balance.
Reactions
Ammonium permanganate is a strong oxidizer due to its permanganate anion, and it is a moderately strong explosive due to the combination of oxidizer permanganate anion and reducing ammonium cation. Dry ammonium permanganate can detonate due to heat, shock, or friction, and it can explode at temperatures above 140 °F (60 °C).
Ammonium permanganate decomposes explosively to manganese dioxide, nitrogen, and water:
2 NH4MnO4 → 2 MnO2 + N2 + 4 H2O
Even at room temperature, ammonium permanganate decomposes slowly in storage. After 3 months, a sample was only 96 percent pure; after 6 months, it had the color of iodine and a strong odor of nitrogen oxides. When decomposed by heat, it emits toxic fumes.
Quaternary ammonium permanganate compounds such as tetrabutylammonium permanganate and benzyltriethylammonium permanganate can be synthesized.