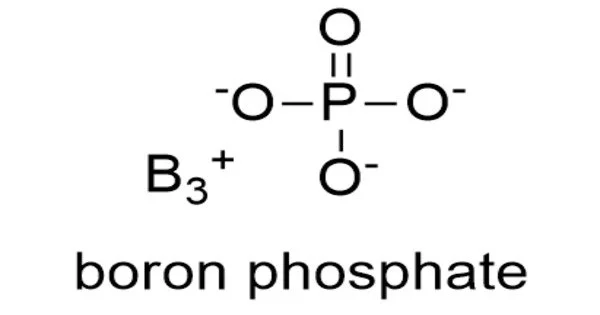
Boron phosphate has the chemical formula BPO4 and is an inorganic compound. The reaction of phosphoric acid and boric acid is the most basic method of producing it. It is a white infusible solid that evaporates at temperatures above 1450 °C.
Boron phosphate is chemically stable and resistant to acids and alkalis, which makes it useful as a protective coating material in corrosive environments. It is a hard and brittle material with a high compressive strength. It also has a low coefficient of thermal expansion, which makes it useful in high-temperature applications where thermal cycling is required.
Properties
- Physical properties: It is a white crystalline solid with a density of 2.54 g/cm³. It is insoluble in water and most organic solvents, but can be dissolved in concentrated acids.
- Thermal stability: It has excellent thermal stability, with a melting point of 950°C and a decomposition temperature of around 1100°C. This makes it useful for high-temperature applications.
- Electrical properties: It is an electrical insulator with a low dielectric constant, which makes it useful for applications where electrical insulation is required.
- Optical properties: It is a transparent material with a refractive index of 1.5, which makes it useful as an optical material for lenses and prisms.
Synthesis
Boron phosphate can be synthesized by a variety of methods, including solid-state reactions, sol-gel methods, and hydrothermal synthesis. Its properties and applications make it a promising material for various technological and industrial applications.
Boron phosphate is synthesized from phosphoric acid and boric acid at a temperature range from 80 °C to 1200 °C. The relatively cold treatment produces a white amorphous powder, which is converted to a microcrystalline product when heated at about 1000 °C for 2 hours.
The main reaction of the process is:
H3BO3 + H3PO4 → BPO4 + 3 H2O
New ways of synthesizing the compound have also been reported, such as hydrothermal and microwave synthesis.
Structure
If obtained at pressure, the ordinary structure is isomorphous with the β-cristobalite, while subjecting it to high pressure is obtained a compound isomorphic with α-quartz. The structure of AlPO4, berlinite, is isomorphous with α-quartz.
Applications
Boron phosphate is used in various applications, including as a catalyst in organic reactions, in the production of ceramics, and as an electrode material in lithium-ion batteries. It also has potential applications in the nuclear industry, as it can absorb neutrons and has a low thermal neutron capture cross-section. It is used as a catalyst for dehydration and other reactions in organic synthesis. Also, it serves as a source of phosphates for exchange reactions in the solid state to obtain metal phosphates.