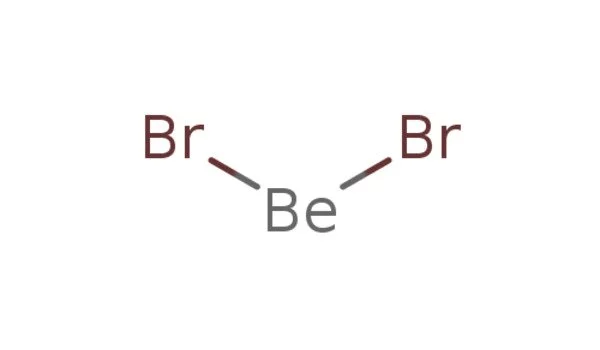
Beryllium bromide is a chemical compound with the formula BeBr2. It is highly hygroscopic and easily dissolves in water. It is an inorganic compound and one of the binary compounds formed by beryllium and a halogen. The compound is a polymer with tetrahedral coordinated Be centers. It is a white solid at room temperature.
Beryllium bromide has a polymeric structure in its solid state. The beryllium atoms are surrounded by four bromine atoms, forming a tetrahedral structure. The compound is typically found as a white solid. It has a high melting point and is soluble in water. In its solid state, the compound has a polymeric structure with Be2+ ions surrounded by bromide ions. It is sensitive to moisture and reacts readily with water to form beryllium hydroxide and hydrogen bromide.
Properties
- Chemical formula: BeBr2
- Molar mass: 168.820 g/mol
- Appearance: colorless white crystals
- Density: 3.465 g/cm3 (20 °C)
- Melting point: 508 °C (946 °F; 781 K)sublimes at 473 °C (883 °F; 746 K)
- Boiling point: 520 °C (968 °F; 793 K)
- Solubility in water: Highly
- Solubility: soluble in ethanol, diethyl ether, pyridine; insoluble in benzene
- Crystal structure: Orthorhombic
Preparation and reactions
It can be prepared by reacting beryllium metal with elemental bromine at temperatures of 500 °C to 700 °C:
Be + Br2 → BeBr2
Beryllium bromide is also formed when treating beryllium oxide with hydrobromic acid:
BeO + 2 HBr → BeBr2 + H2O
It hydrolyzes slowly in water: BeBr2 + 2 H2O → 2 HBr + Be(OH)2
Structure
Two forms (polymorphs) of BeBr2 are known. Both structures consist of tetrahedral Be2+ centers interconnected by doubly bridging bromide ligands. One form consist of edge-sharing polytetrahedra. The other form resembles zinc iodide with interconnected adamantane-like cages.
Uses
Beryllium bromide has limited practical applications. It is primarily used in research and laboratory settings for specific chemical reactions and studies involving beryllium compounds.
Safety
Beryllium compounds are toxic if inhaled or ingested. It can be toxic, and exposure to them should be minimized. Beryllium is known for its toxicity, and inhalation of beryllium dust or fumes can lead to lung diseases.