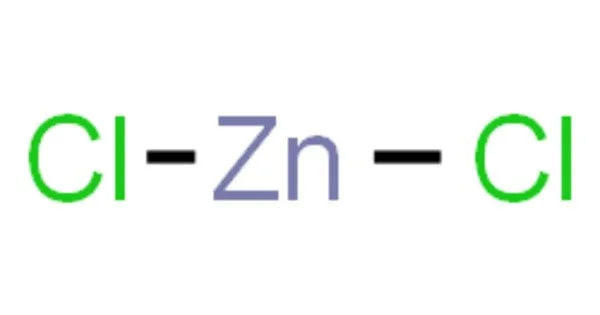The name zinc chloride refers to inorganic chemical compounds with the formula ZnCl2 and their hydrates. Zinc chlorides, which exist in nine crystalline forms, are colorless or white and highly soluble in water.
Zinc chloride has hygroscopic properties, which means it attracts and captures water molecules from its surroundings. This salt is both hygroscopic and deliquescent. Zinc chloride is widely used in textile manufacturing, metallurgical fluxes, and chemical synthesis. As a result, protecting zinc chloride from moisture is critical. Aside from the extremely rare mineral simonkolleite, Zn5(OH)8Cl2•H2O, no mineral with this chemical composition is known.
Properties
- Chemical formula: ZnCl2
- Molar mass: 136.315 g/mol
- Appearance: white crystalline solid; hygroscopic and very deliquescent
- Odor: odorless
- Density: 2.907 g/cm3
- Melting point: 290 °C (554 °F; 563 K)
- Boiling point: 732 °C (1,350 °F; 1,005 K)
- Solubility in water: 432.0 g/ 100 g (25 °C)
- Solubility: soluble in ethanol, glycerol and acetone
- Solubility in ethanol: 430.0 g/100ml

Preparation and purification
The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride. Anhydrous ZnCl2 can be prepared from zinc and hydrogen chloride:
Zn + 2 HCl → ZnCl2 + H2
Hydrated forms and aqueous solutions may be readily prepared similarly by treating Zn metal, zinc carbonate, zinc oxide, and zinc sulfide with hydrochloric acid:
ZnS + 2 HCl + 4 H2O → ZnCl2(H2O)4 + H2S
Unlike many other elements, zinc has only one oxidation state, 2+, making chloride purification easier. Water and hydrolysis products are common impurities in commercial samples of zinc chloride. Recrystallization from hot dioxane may be used to purify such samples. Sublimation in a stream of hydrogen chloride gas, followed by heating the sublimate to 400 °C in a stream of dry nitrogen gas, can be used to purify anhydrous samples. Finally, the most straightforward method involves treating zinc chloride with thionyl chloride.
Role in organic chemistry
Zinc chloride is used as a catalyst or reagent in a wide range of industrial reactions. The main route to benzoyl chloride is the partial hydrolysis of benzal chloride in the presence of zinc chloride. It acts as a catalyst in the production of methylene-bisphenol A. (dithiocarbamate).
The “Lucas reagent,” which consists of hydrochloric acid and ZnCl2, is useful for preparing alkyl chlorides from alcohols. Similar reactions form the basis of industrial routes from methanol and ethanol to methyl chloride and ethyl chloride, respectively.
Applications
Zinc chloride is used as an electrolyte in dry cells. It is used as a disinfectant, condensing agent, dehydrating agent, wood preservative, deodorant, and disinfectant. This compound, when molten, acts as a catalyst for some aromatization reactions. Hexamethyl benzene, for example, can be synthesized from methanol using molten ZnCl2.
Some smoke grenades contain a mixture of zinc oxide and hexachloroethane. When these compounds are ignited, they react to produce zinc chloride smoke, which acts as a smokescreen. Because it forms an easily detectable complex with Ruhemann’s purple, ZnCl2 is also useful in fingerprint detection.
Safety
Zinc chloride is a chemical irritant of the eyes, skin, and respiratory system.