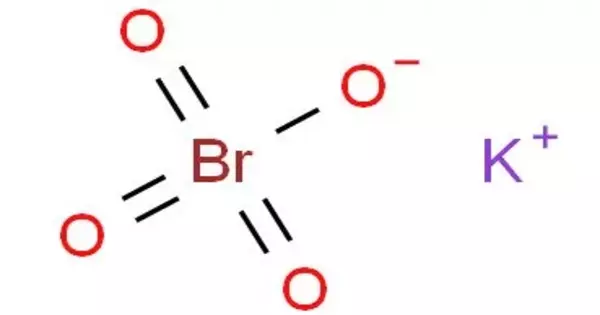
Potassium perbromate is a chemical compound with the formula KBrO4 that is made up of the potassium ion and the perbromate ion. In acid solution, it is a powerful oxidizing agent and a convenient, stable source of bromine. When the Bromide ion is present (either already present in the Bromate solution, as in Bromate-Bromide solutions, or added to the sample before the titration begins) and the solution is acidified.
It is used as an oxidizer to strengthen and increase the elasticity of the dough. It aids in the production of uniformly white bread. It is used to improve the taste of flour. It is used in the manufacturing of malt barley.
Properties
- Chemical formula: KBrO4
- Molar mass: 183 g/mol
- Density: 3.08 g/cm3
- Monoisotopic mass: 181.861694 Da
Preparation
Potassium perbromate can be prepared by reacting perbromic acid with potassium hydroxide:
HBrO4 + KOH → KBrO4 + H2O
It is a powerful oxidizing agent. It is an ionic compound or salt made up of K+ and BrO3-. It is an inorganic substance. It is also known as bromate. It is also water soluble due to its high melting point. It is a moderately toxic substance. It causes nausea, diarrhea, respiratory stimulation, and kidney damage.
Uses
- As a flour and bread improver in baking. It strengthens the dough and allows higher rising.
- In producing malt barley.
- In analytical chemistry.
- Being an effective brominating agent, KBrO3 in sulfuric acid is used to brominate benzene.
Is It Dangerous
If ingested, it is carcinogenic (causes DNA damage), neurotoxic, and nephrotoxic. The compound has been linked to hyperuricemia and thyroid follicular cell tumors. Because of its negative effects, many countries have banned its use as a food additive. Inhalation and contact with the eyes and skin should be avoided because they can cause severe irritation and damage. When it comes into contact with organic material, it can start a fire.