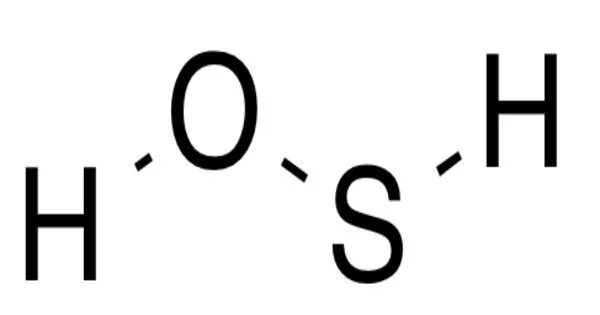The chemical with the formula H-S-O-H is hydrogen thioperoxide, also known as oxadisulfane or sulphur hydride hydroxide. It is the simplest hydrogen chalcogenide containing more than one type of chalcogen and the simple sulfur-substituted analogue of the common hydrogen peroxide (H-O-O-H) chemical.
The chemical has been dubbed the “missing link” between hydrogen peroxide and hydrogen disulfide (H-S-S-H), despite being significantly less stable than either of the other two. It is the inorganic parent structure of the organic compound class sulfenic acid (R-S-O-H) as well as the oxadisulfide linkage (R1-S-O-R2), where “R” can be any organic structure. Sulphur exists in oxidation state 0.
Properties
Hydrogen thioperoxide molecules have a gauche conformation. They are unsymmetrical, but have a low barrier to convert from left-hand to right-hand forms, so that the molecule can tunnel between the forms.
- Chemical formula: H2OS
- Molar mass: 50.08 g·mol−1
- Density: 1.249
- Refractive index (nD): 1.484
Formation
Hydrogen thioperoxide has been synthesised in laboratories through photolysis of an ozone-hydrogen sulphide mixture frozen in argon at 8 K and pyrolysis of di-tert-butyl sulfoxide. Another method is to conduct an electric discharge through water and sulphur.
In the interstellar medium, hydrogen thioperoxide is thought to be formed by the reaction of sulphur monoxide with the trihydrogen cation, dihydrogen, and an electron. Another route is for sulphur monoxide to react with atomic hydrogen to form HOS and HSO, which can then add another hydrogen atom. However, this mechanism is likely to require the removal of excess energy by a dust grain.
Reactions
Two molecules of hydrogen thioperoxide can undergo cyclocondensation to form sulfinothioic acid HS(=O)SH and water.
Hydrosulfide HS− can react with HSOH to yield disulfane HSSH.
Application
- Bleaching Agent: It is a well-known example used as a bleaching agent in the textile and paper industries and as a disinfectant in household applications.
- Oxidizing Agent: It can be used as oxidizing agents in chemical reactions, including the synthesis of various organic compounds.
- Propellant: Some peroxides are used in the production of propellants for rockets and missiles.