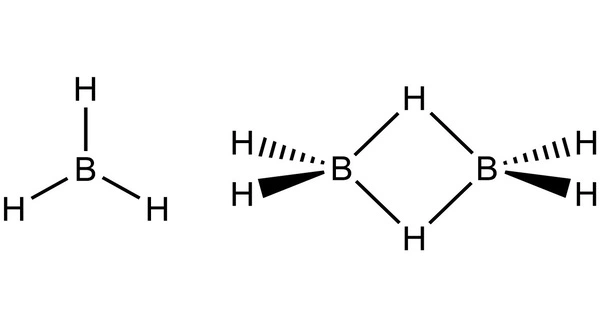
Diborane is an inorganic chemical compound with the formula B2H6 that is composed of boron and hydrogen. It is a toxic, colorless, pyrophoric gas with a repulsively sweet odor. Borethane, boron hydride, and diboron hexahydride are some synonyms. Diborane is a vital boron compound with numerous applications. Its electronic structure has gotten a lot of attention. Its derivatives are useful reagents.
Diborane was first synthesized in the nineteenth century through the hydrolysis of metal borides, but it was never studied. Alfred Stock, a major pioneer in the chemistry of boron hydrides, conducted research that led to methods for the synthesis and handling of the highly reactive, volatile, and often toxic boron hydrides from 1912 to 1936. He proposed the first diborane-like ethane structure. S. H. Bauer’s electron diffraction measurements initially appeared to support his proposed structure.
Properties
- Chemical formula: B2H6
- Molar mass: 27.67 g·mol-1
- Appearance: Colorless gas
- Odor: repulsive and sweet
- Density: 1.131 g/L
- Melting point: −164.85 °C (−264.73 °F; 108.30 K)
- Boiling point: −92.49 °C (−134.48 °F; 180.66 K)
- Solubility in water: Reacts
- Solubility in other solvents: Slightly soluble in Diglyme and Diethyl Ether,
- Vapor pressure: 39.5 atm (16.6 °C)
Reactions
- Air, water, oxygen
As a pyrophoric substance, diborane reacts exothermically with oxygen to form boron trioxide and water:
2 B2H6 + 6 O2 → 2 B2O3 + 6 H2O (ΔHr = −2035 kJ/mol = −73.47 kJ/g)
Diborane reacts violently with water to form hydrogen and boric acid:
B2H6 + 6 H2O → 2 B(OH)3 + 6 H2 (ΔHr = −466 kJ/mol = −16.82 kJ/g)
Diborane also reacts with alcohols similarly. Methanol for example give hydrogen and trimethylborate:
B2H6 + 6 MeOH → 2 B(OMe)3 + 6 H2
- Lewis acidity
The formation of adducts with Lewis bases is a common reaction pattern. Such initial adducts frequently give rise to other products. For example, borane-tetrahydrofuran degrades to borate esters after acting similarly to diborane. Its adduct with dimethyl sulfide is useful in organic synthesis.
With ammonia diborane, the diammoniate of diborane, DADB, is formed, with small amounts of ammonia borane as a byproduct. The ratio depends on the conditions.
- Hydroboration
Diborane readily reacts with alkenes in the hydroboration reaction to form trialkylboranes. This reaction pattern is fairly general, and the alkyl borates formed are easily derivatized, for example, to alcohols. Although diborane was used in early hydroboration research, it has since been replaced by borane dimethylsulfide, which is safer to handle.
Uses
Diborane has been tested as a rocket propellant due to the exothermicity of its reaction with oxygen. Complete combustion is strongly exothermic. However, some boron monoxide, B2O, is produced during combustion in the rocket engine. This conversion is analogous to the incomplete combustion of hydrocarbons, which results in the production of carbon monoxide (CO). Diborane was also difficult to manage.
Diborane has been investigated as a precursor to metal boride films and for the p-doping of silicon semiconductors.
Safety
Diborane is a flammable gas. In most cases, commercially available adducts are used instead, at least in organic chemistry. Borane-tetrahydrofuran (borane-THF) and borane-dimethylsulfide are two of these adducts. The toxic effects of diborane are mitigated because the compound is so unstable in air. The toxicity to laboratory rats has been studied.