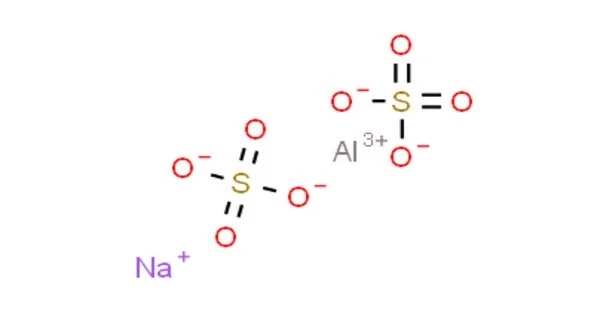
Sodium aluminium sulfate is the inorganic compound with the chemical formula NaAl(SO4)2·12H2O. It is a white, odorless crystalline powder that dissolves readily in water. It is stable under normal conditions of use and storage. Also known as soda alum, sodium alum, or SAS, this white solid is used in the manufacture of baking powder and as a food additive. Its official mineral name is alum-Na.
Properties
Sodium aluminum sulfate, like its potassium analog, crystallizes as the dodecahydrate in the classical cubic alum structure. Sodium alum is extremely soluble in water and difficult to purify. It is preferable to mix the component solutions in the cold and evaporate them at a temperature not exceeding 60 °C when making this salt. At 0 °C, 100 parts water dissolve 110 parts sodium alum, and 51 parts dissolve at 16 °C.
- Chemical formula: NaAl(SO4)2·12H2O
- Molar mass: 458.28 g/mol
- Appearance: white crystalline powder
- Density: 1.6754 (20 °C)
- Melting point: 61 °C (142 °F; 334 K)
- Solubility in water: 208 g/100 ml (15 °C)
- Crystal structure: Cubic, cP96

Production and natural occurrence
Sodium aluminum sulfate is produced by combining sodium sulfate and aluminium sulfate. An estimated 3000 ton/y (2003) are produced worldwide.
The dodecahydrate is known in mineralogy as alum-(Na). Two other rare mineral forms are known: mendozite (undecahydrate) and tamarugite (hexahydrate).
Application
It is commonly used as a food additive, where it is listed as E number E522. It is used in water treatment as a coagulant to help remove suspended solids and turbidity. In addition, it is used in the paper industry as a sizing agent to improve the strength and water resistance of paper.
However, it is important to note that excessive consumption of sodium aluminum sulfate can be harmful to human health. In particular, it has been linked to neurological disorders such as Alzheimer’s disease, although the evidence for this association is not conclusive. Therefore, it is important to use sodium aluminum sulfate in moderation and as directed by professionals in the relevant industries.
Toxicity
Sodium aluminium sulfate is generally considered safe for consumption in food and has no known toxic effects in humans when used in normal amounts. However, excessive consumption can lead to gastrointestinal upset.