Common Ion Effect
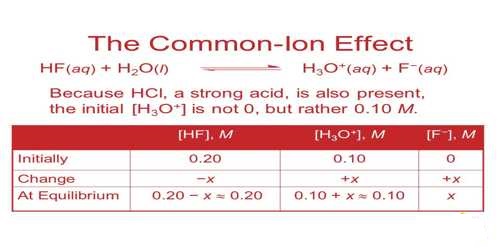
The dissociation of an electrolyte may be depressed by adding another electrolyte having an ion (cation or anion) in common with the dissolved electrolyte. Common ion effect describes the suppressing effect on ionization of an electrolyte when another electrolyte is added that shares a common ion.
The phenomenon in which the dissociation of a weak electrolyte is suppressed due to the presence of another electrolyte having an ion in common with the dissolved weak electrolyte is known as “The Common Ion Effect”.
This may be illustrated with ethanoic acid and sodium ethanoate. In solution the following equilibrium is established:
CH3COOH (aq) ↔ CH3COO– (aq) + H+ (aq)
If CH3OONa is added to the solution of ethanoic acid, it dissociates completely, since it is a strong electrolyte.
CH3OONa (s) + aq → CH3COO– (aq) + Na+ (aq)
According to Le Chatelier’s principle addition of the common ion CH3COO– from CH3OONa to the solution of CH3COOH will shift the equilibrium to the left and thereby suppress the dissociation of CH3COOH. As a result the solution will be less acidic than the solution having the same concentration of CH3COOH. The common ion effect plays an important role in determining the pH of a solution as well as the solubility of sparingly soluble salts.