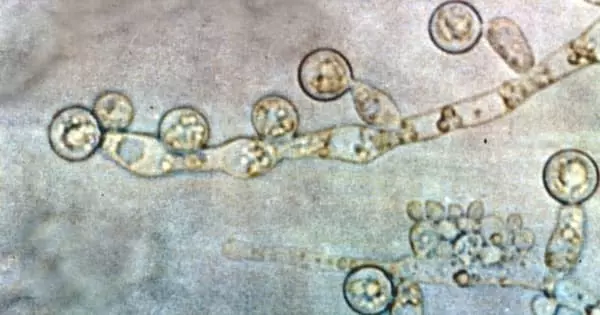
Scientists identified some of the key proteins in Candida albicans biofilms that control both how they resist antifungal drugs and how they spread throughout the body in a new study designed to better understand and combat these structures.
The microbes that make us sick frequently find ways to avoid our attacks. The biofilm matrix, a sticky, armor-like goo that encases clusters of disease-causing organisms, is perhaps the most important of these strategies.
This defense works, albeit in tragic ways at times. Biofilms, for example, form quickly and invisibly on medical devices such as catheters and implants and are highly resistant to drugs that could otherwise treat them. Infections caused by them cost tens of thousands of lives and billions of dollars in the United States each year.
“There are no antimicrobials that have been approved for the treatment of biofilms. A biofilm can only be treated by physically removing it from the body” David Andes, a medical professor at the University of Wisconsin School of Medicine and Public Health, agrees.
Andes and his colleagues identified some of the key proteins in Candida albicans biofilms that control both how the fungus resists antifungal drugs and how it spreads throughout the body in a new study designed to better understand and combat these structures.
Biofilms are composed of a complex soup of materials secreted by individual cells within them, such as proteins. There are no antimicrobials that have been approved for the treatment of biofilms. A biofilm can only be treated by physically removing it from the body.
David Andes
While more research is needed, the newly discovered proteins represent potential drug targets for impairing a pathogen’s antimicrobial defenses. In fact, the study discovered that Candida strains that couldn’t produce some of these proteins were much more sensitive to the antifungal fluconazole.
In a rat infection model, however, interfering with some of these same proteins increased the likelihood of biofilms spreading to the kidney. This is a flaw that will require further investigation. Andes and his colleagues, which included Aaron Mitchell, a biology professor at the University of Georgia, published their findings in the journal Nature Communications.
Candida is a mysterious organism. The fungus frequently lives in and on healthy people, causing no harm. However, it can easily infect immunocompromised people, as well as those who are otherwise healthy.
Biofilms are composed of a complex soup of materials secreted by individual cells within them, such as proteins. The researchers screened hundreds of these proteins using a machine-learning algorithm to identify likely candidates involved in biofilm production and function. They found 63 proteins to look into further. In lab tests, 13 of the Candida mutants that were unable to produce these proteins became more susceptible to the antifungal fluconazole.
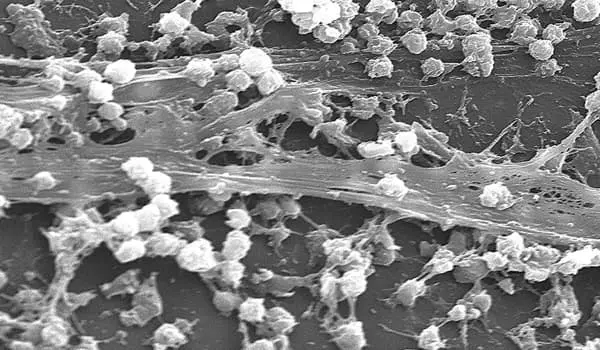
The proteins were also studied in a rat model involving venous catheters by the Andes lab, which specializes in developing animal models to test drug resistance. These catheters, which are inserted into large veins, are frequently left in place for months to aid in drug delivery, such as during chemotherapy treatment. Catheters are prone to infection because they stay in the body for an extended period of time.
When the researchers tested four of the 13 drug-sensitive fungal mutants in the rat model, they found that all four were still susceptible to fluconazole, as previous lab tests had shown. While the antifungal has little effect on normal Candida biofilms, it reduces mutant fungal populations by a factor of 30 or more.
Biofilms are responsible for more than just drug resistance. They have an impact on a pathogen’s entire lifecycle. “Dispersion is the final stage of a biofilm’s lifecycle. Cells leave the biofilm and spread throughout the body “Andes says. This body-wide dispersal greatly increases the risk from infections.
The researchers discovered 17 mutants that influenced the dispersion process; the majority of them dispersed more easily. When three of these high-dispersal mutants were tested in rats, they caused a more than 10-fold increase in the spread of Candida to the kidneys.
Surprisingly, two of the mutants were both more susceptible to antifungals and more likely to spread to the kidney, indicating a mixed bag of clinical outcomes. According to Andes, the proteins’ overlapping functions – partly antimicrobial, partly controlling dispersal – indicate that they play complex roles in biofilms.
The Andes lab has already identified a drug that can disrupt the fungus’ defenses. They recently discovered that the antifungal turbinmicin, discovered by Andes and his colleagues in 2020, can inhibit Candida’s ability to secrete these proteins and other biofilm components, making the pathogen more susceptible to drugs.