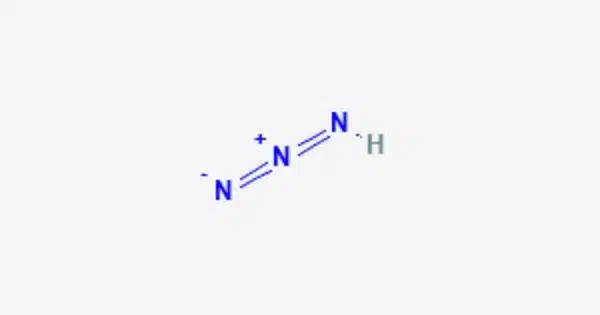
Hydrazoic acid (also known as hydrogen azide or azoimide) is a chemical compound having the formula HN3. At room temperature, it is a highly hazardous and volatile liquid, although it can also exist as a colorless gas. At room temperature and pressure, it is a colorless, volatile, and explosive liquid. It is a pnictogen hydride because it is a nitrogen and hydrogen compound.
The nitrogen atoms in hydrazoic acid have a fractional oxidation state of -1/3. Theodor Curtius isolated it for the first time in 1890. Although the acid has few applications, its conjugate base, the azide ion, is useful in a variety of procedures.
Properties
It is a white liquid with a strong, disagreeable odor in its pure form. It is highly flammable, and inhaling its fumes can be hazardous. It can also combine to generate explosive compounds. It is exceedingly hazardous to humans and, if consumed, inhaled, or absorbed via the skin, can be fatal. It can have an impact on the central nervous system and cause respiratory, cardiovascular, and neurological issues.
- Chemical formula: HN3
- Molar mass: 43.029 g·mol−1
- Appearance: colorless, highly volatile liquid
- Density: 1.09 g/cm3
- Melting point: −80 °C (−112 °F; 193 K)
- Boiling point: 37 °C (99 °F; 310 K)
- Solubility in water: highly soluble
- Solubility: soluble in alkali, alcohol, ether
- Acidity (pKa): 4.6
- Conjugate base: Azide
- Molecular shape: approximately linear
Preparation
It can be prepared by various methods, including the reaction of sodium azide (NaN3) with a strong acid. The resulting solution contains hydrazoic acid as well as other products.
Applications
Hydrazoic acid is largely utilized in chemical synthesis and research. It is also utilized in several industrial processes, including as the synthesis of specific chemicals and as an explosive detonator.
Furfural was treated with a mixture of hydrazoic acid (HN3) and perchloric acid (HClO4) in the presence of magnesium perchlorate in benzene solution at 35 °C to produce 2-furonitrile, a pharmacological intermediate and potential artificial sweetening agent.
When it comes into touch with heavy metals, it can generate explosive compounds. To avoid mishaps, it should be stored and handled with care.
Toxicity
Hydrazoic acid is highly poisonous and highly volatile. It has a strong odor and its vapor can produce severe headaches. The substance is a non-accumulative toxin. It should be handled with utmost caution due to its severe toxicity and instability. When dealing with this substance, proper protection equipment and ventilation are required.