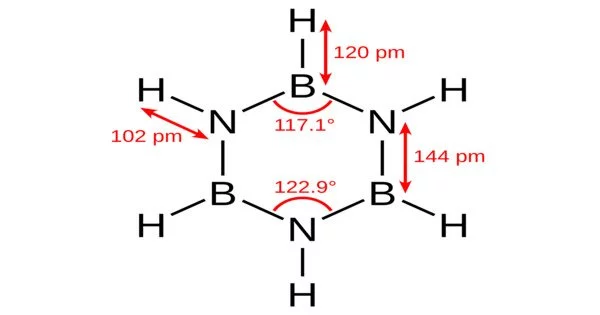
Borazine is an inorganic compound made up of boron, nitrogen, and hydrogen. Borazole, or B3H6N3, is a non-polar inorganic compound with the chemical formula B3H6N3. The three BH units and three NH units alternate in this cyclic compound. With benzene, the compound is isoelectronic and isostructural. As a result, borazine is also known as “inorganic benzene.” Borazine, like benzene, is a colorless liquid with an aromatic odor.
Properties
Borazine is a colourless liquid with an aromatic smell. In water it decomposes to boric acid, ammonia, and hydrogen. Borazine, with a standard enthalpy change of formation ΔHf of -531 kJ/mol, is thermally very stable.
- Chemical formula: B3H6N3
- Molar mass: 80.50 g/mol
- Appearance: Colorless liquid
- Density: 0.81 g/cm3
- Melting point: −58 °C (−72 °F; 215 K)
- Boiling point: 53 °C (127 °F; 326 K) (55 °C at 105 Pa)
Structure
The structure is isoelectronic and isostructural with benzene and for this reason borazine is called inorganic benzene by a proposal of Nils Wiberg and the compound also goes by the name of borazol from the German name for benzene which is benzol. It is isostructural with benzene and bond lengths are identical just as in benzene. The distance between boron and nitrogen in the ring is 0.1436 nm, the carbon carbon bond in benzene has a length of 0.1397 nm.
Preparation
Borazine can be prepared by heating together boron trichloride and ammonium chloride. The initial product formed is trichloroborazine which on reducing with sodium borohydride yields borazine.
Synthesis
The compound was reported in 1926 by the chemists Alfred Stock and Erich Pohland by a reaction of diborane with ammonia.
Borazine can be synthesized by treating diborane and ammonia in a 1:2 ratio at 250–300 °C with a conversion of 50%.
3 B2H6 + 6 NH3 → 2 B3H6N3 + 12 H2
An alternative more efficient route begins with sodium borohydride and ammonium sulfate:
6 NaBH4 + 3 (NH4)2SO4 → 2 B3N3H6 + 3 Na2SO4 + 18 H2
In a two-step process to borazine, boron trichloride is first converted to trichloroborazine:
3 BCl3 + 3 NH4Cl → Cl3B3H3N3 + 9 HCl
The B-Cl bonds are subsequently converted to B-H bonds:
2 Cl3B3H3N3 + 6 NaBH4 → 2 B3H6N3 + 3 B2H6 + 6 NaCl
Aromaticity
A number of computational and experimental studies of borazine’s aromaticity have been conducted due to its similarities to benzene. The number of pi electrons in borazine follows the 4n + 2 rule, and the lengths of the B-N bonds are equal, indicating that the compound may be aromatic. However, the difference in electronegativity between boron and nitrogen results in unequal charge sharing, resulting in bonds with greater ionic character, and thus it is expected to have poorer electron delocalization than the all-carbon analog.
- Natural bond orbitals (NBO)
Natural bond orbital (NBO) analysis of borazine indicates that it has low aromaticity. In the NBO model, B-N bonds in the ring are slightly displaced from the nuclear axes, and B and N have large charge differences. Natural chemical shielding (NCS) analysis adds to the evidence for aromaticity by demonstrating the contribution of the B-N bond to magnetic shielding.
- Electron localization function (ELF)
The electron localization function (ELF) topological analysis of bonding in borazine indicates that borazine is an aromatic compound. However, due to differences in electron basin bifurcation values, the bonding in borazine is less delocalized than that in benzene.
Applications
Borazine and its derivatives have the potential to be precursors to boron nitride ceramics. Boron nitride can be made by heating polyborazylene to 1000 degrees Celsius. Borazines are also potential starting materials for other ceramics such as boron carbonitrides:
Borazine can also be used as a precursor to grow boron nitride thin films on surfaces, such as the rhodium nanomesh structure.