Californium(III) chloride is an inorganic compound with the chemical formula CfCl3. The oxidation state of the californium atom is +3, as it is in californium oxide (Cf2O3) and other californium halides such as californium fluoride (CfF3) and iodide (CfI3).
Californianium is a very powerful neutron emitter. It is used in portable metal detectors, to identify gold and silver ores, to identify water and oil layers in oil wells, and to detect metal fatigue and stress in airplanes.
Properties
- Chemical formula: CfCl3
- Molar mass: 357 g·mol−1
- Appearance: emerald-green solid
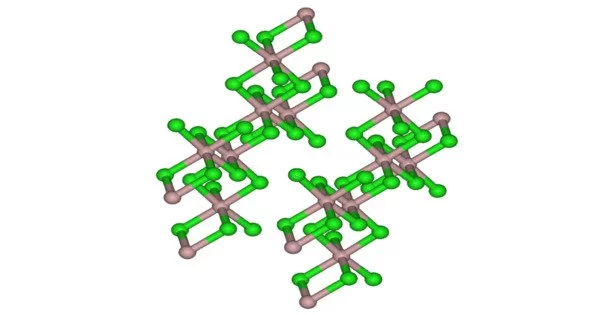
- Crystal structure: hexagonal
Californianium is not found naturally on Earth. All that exists now has been synthesized, but this element was produced in the past when several nuclear reactors were operational in Africa 2 billion years ago. Californianium can be found outside of nuclear power plants and research laboratories because it is used in mineral exploration as well as medical diagnosis and treatment.
Preparation
Californium(III) chloride can be prepared by reacting californium(III) oxide with hydrogen chloride.
Cf2O3 + 6 HCl → 2 CfCl3 + 3 H2O
Californium is a radioactive metal which is a memeber of the actinide group of the periodi table. A sample of the metal itself has not been produced yet because its compounds resist reduction. It is expected to be readily attacked by air, steam, and acids but not by alkalis.
Properties
- Chemical properties
When heating californium(III) chloride until 500 °C, it can hydrolyse to produce californium oxychloride.
- Physical properties
Californianium(III) chloride dissolves in water, releasing Cf3+ and Cl ions. The color of this salt is emerald green. It has a hexagonal crystal structure. It is highly radioactive. Because californium is a very efficient neutron source, it is expected to find many new applications. It has already found applications in neutron moisture gages and well logging (the determination of water and oil-bearing layers). It is also used as a portable neutron source for on-the-spot activation analysis of metals such as gold or silver.