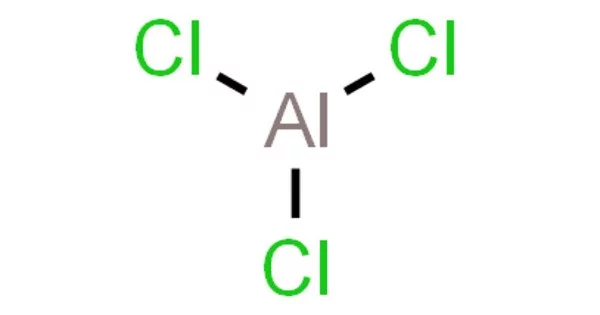
Aluminium chloride (AlCl3) is a chemical compound that is white or yellowish in color and exists as a solid at room temperature. It is soluble in water and commonly used as a Lewis acid in organic synthesis and as a catalyst for various chemical reactions. Aluminium chloride is also used in the production of aluminium metal, as a flocculating agent in water treatment, and in deodorants and antiperspirants.
It is an inorganic compound. It forms hexahydrate with the formula [Al(H2O)6]Cl3, containing six water molecules of hydration. Both are colorless crystals, but samples are often contaminated with iron(III) chloride, giving a yellow color.
Commercially, anhydrous material is important. Its melting and boiling points are both low. It is primarily produced and consumed in the production of aluminum metal, but it is also used extensively in other areas of the chemical industry. The compound is frequently referred to as a Lewis acid. At low temperatures, it is an example of an inorganic compound that reversibly changes from a polymer to a monomer.
Properties
It is a chemical compound that is a white crystalline solid. It is used in the production of aluminum metal, as a catalyst in organic synthesis, and as an antiperspirant in personal care products. It is highly hygroscopic and soluble in water, but reacts with moisture to form hydrochloric acid.
- Chemical formula: AlCl3
- Molar mass: 133.341 g/mol (anhydrous); 241.432 g/mol (hexahydrate)
- Appearance: Colourless crystals, hygroscopic
- Density: 2.48 g/cm3 (anhydrous); 2.398 g/cm3 (hexahydrate)
- Melting point: 100 °C (212 °F; 373 K) (hexahydrate, decomposes)
- Solubility in water: 439 g/L (0 °C); 490 g/L (100 °C)
- Solubility: Soluble in hydrogen chloride, ethanol, chloroform, carbon tetrachloride
- Slightly soluble in benzene
- Crystal structure: Monoclinic, mS16

Synthesis
Aluminium chloride is manufactured on a large scale by the exothermic reaction of aluminium metal with chlorine or hydrogen chloride at temperatures between 650 to 750 °C (1,202 to 1,382 °F).
2 Al + 3 Cl2 → 2 AlCl3
2 Al + 6 HCl → 2 AlCl3 + 3 H2
Aluminium chloride may be formed via a single displacement reaction between copper(II) chloride and aluminium metal.
2 Al + 3 CuCl2 → 2 AlCl3 + 3 Cu
In 1993, approximately 21,000 tons were produced in the United States, not including the amounts consumed in the production of aluminum.
Aluminium oxides are dissolved in hydrochloric acid to produce hydrated aluminium trichloride. Metallic aluminum readily dissolves in hydrochloric acid, releasing hydrogen gas and producing significant heat. When heated, this solid does not produce anhydrous aluminium trichloride; instead, the hexahydrate decomposes to aluminium hydroxide:
[Al(H2O)6]Cl3 → Al(OH)3 + 3 HCl + 3 H2O
Aluminium also forms a lower chloride, aluminium(I) chloride (AlCl), but this is very unstable and only known in the vapour phase.
Natural occurrence
Anhydrous aluminum chloride is not found in nature. The hexahydrate, on the other hand, is known as the rare mineral chloraluminite. Cadwaladerite is a more complex, basic, and hydrated aluminum chloride mineral.
Safety
Because anhydrous AlCl3 reacts violently with bases, precautions must be taken. If inhaled or come into contact with it, it can irritate the eyes, skin, and respiratory system.