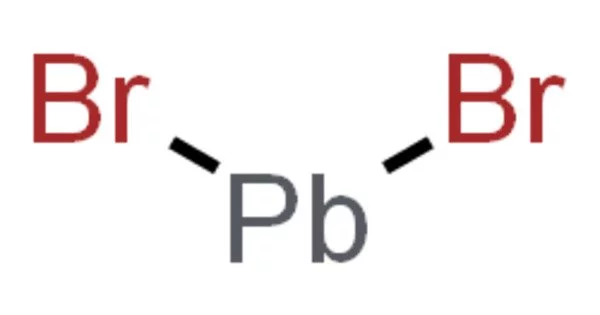
The inorganic compound with the formula PbBr2 is lead(II) bromide. It looks like white powder. It is produced during the combustion of standard leaded gasolines. It is used in photography to develop images, as an inorganic filler in fire-resistant plastics, as a photopolymerization catalyst for acrylamide monomer, and as a welding flux to join aluminum or its alloys to other metals.
Properties
White orthorhombic crystals; density 6.66 g/cm3; melts at 373°C; forms a horn-like mass on solidification; vaporizes at 916°C; decomposes slowly on exposure to light; sparingly soluble in cold water (4.55 g/L at 0°C and 8.44 g/L at 20°C, respectively); moderately soluble in boiling water (44.1g/L at 100°C); Ksp 6.60×10-6 at 25°C; insoluble in alcohol; slightly soluble in ammonia; soluble in alkalies and also in sodium or potassium bromide solutions.
- Chemical formula: PbBr2
- Molar mass: 367.01 g/mol
- Appearance: white powder
- Density: 6.66 g/cm3
- Melting point: 370.6 °C (699.1 °F; 643.8 K)
- Boiling point: 916 °C (1,681 °F; 1,189 K) (vaporizes)
- Solubility in water: 0.455 g/100 mL (0 °C); 4.41 g/100 mL (100 °C)
- Solubility: insoluble in alcohol; soluble in ammonia, alkali, KBr, NaBr
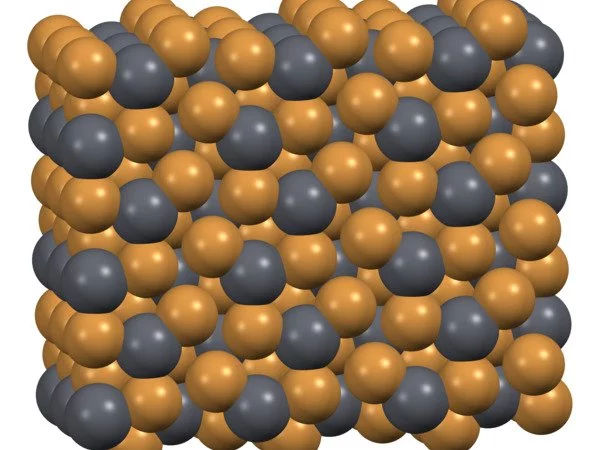
Preparation
It is typically made by treating lead salt solutions (e.g., lead(II) nitrate) with bromide salts. This method takes advantage of its low water solubility – only 0.455 g dissolves in 100 g of water at 0 °C. In boiling water, it is about ten times more soluble.
PbBr2 and lead chloride (cotunnite) have the same crystal structure – they are isomorphous. Pb2+ is surrounded by nine Br ions in a distorted tricapped trigonal prismatic geometry in this structure. Seven of the Pb-Br distances are shorter, in the range 2.9-3.3 Å, while two of them are longer at 3.9 Å. The coordination is therefore sometimes described as (7+2).
Lead bromide is prepared by treating an aqueous solution of lead nitrate with hydrobromic acid or with sodium or potassium bromide:
Pb2+ + 2Br¯ → PbBr2
The solution is allowed to stand to let the precipitate settle.
The compound also may be obtained by adding lead carbonate or lead monoxide to hydrobromic acid.
As a result of the use of leaded gasoline, lead bromide was prevalent in the environment. Tetraethyl lead was once widely used to improve gasoline combustion properties. To keep the resulting lead oxides from fouling the engine, gasoline was treated with an organobromine compound, which converted the lead oxides into the more volatile lead bromide, which was then exhausted from the engine and released into the environment.
Safety
Lead(II) bromide, like other lead compounds, is classified as probably carcinogenic to humans (Category 2A) by the International Agency for Research on Cancer (IARC). Its environmental release as a byproduct of leaded gasoline was highly contentious.