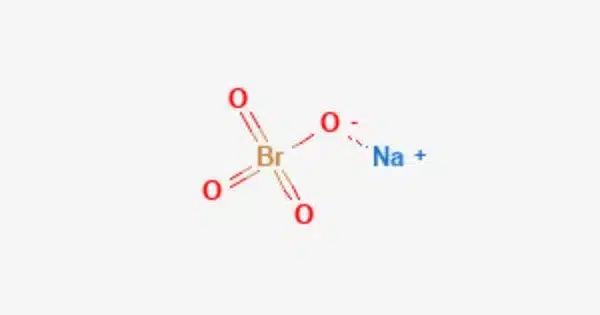
Sodium perbromate is a chemical compound composed of the sodium ion and the perbromate ion, with the chemical formula NaBrO4. It is a powerful oxidizing agent and is highly reactive. Like other perbromates, it contains the bromine atom in its highest oxidation state, which is +7.
Properties
Sodium perbromate is typically a white crystalline solid. It is soluble in water, and the resulting solution is highly alkaline. It is a strong oxidizing agent, and it can react vigorously with reducing agents. It can be used in various chemical reactions to oxidize other compounds.
- Chemical formula: NaBrO4
- Molar mass: 166.89 g mol−1
- Solubility in water: very soluble
- Density: 2.57 g/cm3
- Melting point: 266 °C
Preparation
Sodium perbromate can be prepared by reacting sodium bromate with fluorine and sodium hydroxide:
NaBrO3 + F2 + 2 NaOH → NaBrO4 + 2 NaF + H2O
Application
Sodium perbromate has limited practical applications but can be used in some chemical synthesis processes where a strong oxidizing agent is needed. It is not as commonly encountered as other bromine compounds, such as sodium bromide or sodium bromate, in everyday life or industrial applications. Its primary use is in specialized chemical reactions where a high degree of oxidation is required.
Safety Precautions
Due to its strong oxidizing properties, sodium perbromate should be handled with care. It can pose safety hazards and should be stored and used in a controlled manner.