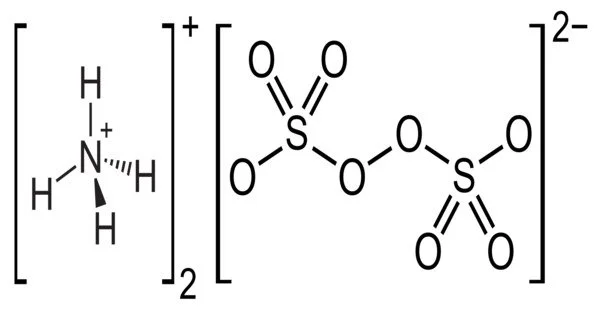
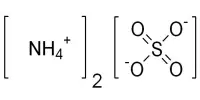The inorganic compound ammonium persulfate (APS) has the formula (NH4)2S2O8. It is a colorless (white) salt that is much more soluble in water than the related potassium salt. It is highly soluble in water and a powerful oxidizing agent. It is a strong oxidizing agent used in polymer chemistry, as an etchant, as a cleaning and bleaching agent, and as a cleaning and bleaching agent. It is used in polymer chemistry as a bleaching agent, cleaning agent, and etchant, among other things.
The salt dissolves in water in an endothermic process. It has numerous applications in polymer chemistry, including bleaching, cleaning, and etchanting.
Properties
Ammonium persulfate is a white crystalline solid. It has a high oxidizing power. It is a colorless salt that is far more soluble in water than the related potassium salt. It is a strong oxidizing agent that is used in polymer chemistry, as a cleaning and bleaching agent, and as an etchant.
- Chemical formula:(NH4)2S2O8
- Molar mass: 228.18 g/mol
- Appearance: white to yellowish crystals
- Density: 1.98 g/cm3
- Melting point: 120 °C (248 °F; 393 K) decomposes
- Solubility in water: 80 g/100 mL (25 °C)
- Solubility: Moderately soluble in MeOH

Preparation
Ammonium persulfate is made by electrolysis at a high current density of a cold concentrated solution of either ammonium sulfate or ammonium bisulfate in sulfuric acid. Hugh Marshall was the first to describe the method.
It is made by electrolyzing a cold concentrated solution of either ammonium sulfate or ammonium bisulfate in the presence of sulfuric acid at a very high current density.
Uses
Ammonium persulfate is a common component of hair bleach. APS has many commercial applications due to its use as an oxidizing agent and a source of radicals. In organic chemistry, persulfates are used as oxidants. In the Minisci reaction, for example.
Ammonium persulfate is used as a bleaching agent, as an initiator in olefin polymerization, in photography, hair bleach, and batteries. It is also employed in the cleaning of infected yeast, food preservatives, and printed circuit boards.
- It is used in the printed circuit boards.
- Used in the olefin polymerization as an initiator.
- Used for photography.
- Used as an additive for preserving the food.
- Used as an oxidising agent.
- Used to wash the infected yeast.
- Used for removing the pyrogallol stains.
- Used as a depolarizer in batteries.
- Used as a common ingredient in the hair bleaches.
Safety
Contact with ammonium persulfate-containing airborne dust may cause irritation to the eyes, nose, throat, lungs, and skin. Breathing difficulties may occur if exposed to high levels of dust.
Persulfate salts have been identified as a major cause of asthmatic effects. Furthermore, exposure to ammonium persulfate has been linked to asthmatic symptoms in hairdressers and receptionists working in the industry. These asthmatic effects are thought to be caused by the oxidation of cysteine and methionine residues.