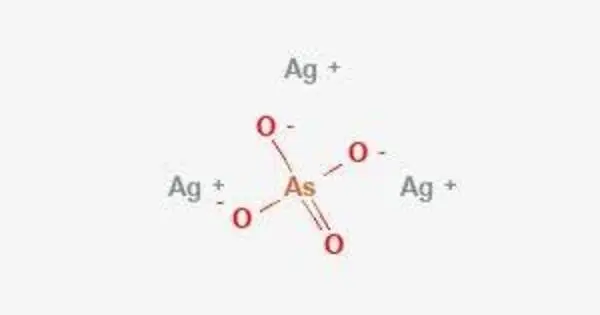
Silver arsenate is an inorganic compound with the formula Ag3AsO4. It has been used in qualitative analysis to distinguish between phosphate (Ag3PO4 is yellow) and arsenate(V) solutions. The compound may appear as a white or yellowish solid.
Silver arsenate is likely to be sparingly soluble in water, as many silver salts exhibit limited solubility in aqueous solutions. It may be reactive, and its properties may vary depending on the environmental conditions. Arsenic compounds, in general, can be toxic, so it is crucial to handle silver arsenate with care. Proper safety precautions should be taken, and exposure should be minimized.
Properties
It is an inorganic compound and is typically encountered in the form of its salt, which is produced by the reaction between silver compounds and arsenic-containing compounds.
- Molar mass: 462.52 g/mol
- Appearance: brown powder/lumps
- Density: 6.657 g/cm3
- Melting point: 830 °C (1,530 °F; 1,100 K) (decomposes)
- Solubility in water: 0.64 mg/L
- Solubility product (Ksp): 1.03×10−22
- Solubility: soluble in acid, aqueous ammonia
- Crystal structure: cubic
Uses
Silver arsenate doesn’t have significant practical uses on its own. However, silver compounds, in general, have been historically used for various applications, including photography, silver plating, and antimicrobial purposes. The use of arsenic compounds in general is limited due to the toxicity of arsenic.
Toxicity
Arsenic is a known toxic substance, and exposure to arsenates, including silver arsenate, can be harmful. Ingestion or inhalation of arsenic compounds can lead to serious health issues. Therefore, proper precautions and safety measures should be taken when handling such substances.