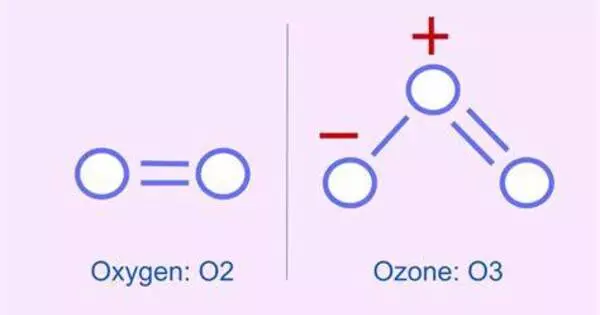
Protonated ozone is a molecule formed by adding a proton (H+) to an ozone molecule (O3). It is a hydrogen polyoxide with the molecular formula HO+3 (also written O3H+). It is a cationic structure composed of an ozone unit with a hydrogen atom attached to one end. This substance is thought to exist as an intermediate in a variety of interstellar, atmospheric, and synthetic chemical processes.
The addition of a proton to a molecule results in the formation of a positively charged species. It was created in mass spectrometer experiments by protonating ozone with various strong acids. It has been used as a precursor in other experiments to generate hydrogen ozonide.
Protonated ozone is a molecular species formed by the addition of a proton (H+) to ozone (O3). Depending on the atom arrangement, the chemical formula for protonated ozone can be written as HOO2 or H2O3. This process usually takes place in the presence of acidic conditions.
Properties
- Chemical formula: HO3+
- Molar mass: 49.004 g·mol−1
- Conjugate base: Ozone
- Structure: The addition of a proton to ozone may cause changes in its molecular structure, such as changes in bond lengths and angles. The precise geometry will be determined by the protonation method.
- Stability: Temperature, pressure, and the surrounding environment all influence the stability of protonated ozone. It could be a reactive species that participates in chemical reactions.
- Ionization Energy: Because removing an electron from a positively charged species requires more energy, it will have a higher ionization energy than neutral ozone.
Reactivity
It may be reactive and can participate in various chemical reactions. The positive charge on the molecule makes it susceptible to interactions with negatively charged species. The reaction can be represented as follows:
O3 + H+ → HOO+
In this reaction, ozone (3O3) reacts with a proton (H+) to form the protonated ozone species (HOO+). Protonated ozone is a transient species and is often involved in atmospheric chemistry, particularly in processes related to acid rain and certain reactions in the upper atmosphere.