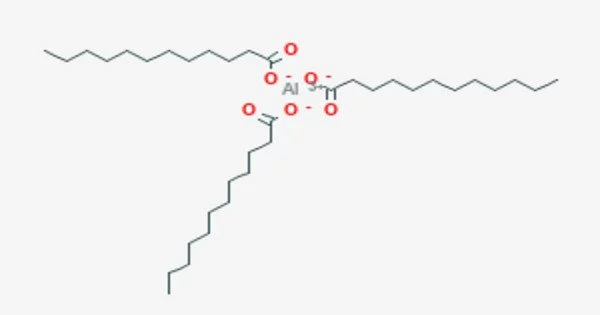
Aluminum laurate is a metal-organic compound with the chemical formula C36H69AlO6. It is a chemical compound that consists of aluminum cations (Al3+) and laurate anions (C11H23O2-). The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid (lauric acid). It is an aluminum salt of lauric acid, which is a saturated fatty acid commonly found in coconut oil and palm kernel oil.
Aluminum laurate is an organic compound that is derived from lauric acid, a fatty acid found in coconut oil and palm kernel oil. It is commonly used as an emulsifying and thickening agent in personal care products and cosmetics.
Physical properties
Aluminum laurate is a white or off-white powder. It is insoluble in water but soluble in organic solvents such as ethanol and propylene glycol. It is an effective emulsifying agent, meaning that it can help to mix two substances that normally do not mix, such as oil and water.
- Chemical formula: C36H69AlO6
- Molar mass: 624.9
- Appearance: White powder
- Boiling point: 296 °C (565 °F; 569 K)
- Solubility in water: Soluble
Preparation
It can be prepared by reacting aluminum chloride with sodium laurate or potassium laurate. Here’s one possible method for preparing aluminum laurate:
Materials: Aluminum chloride (AlCl3), Sodium laurate or potassium laurate, Anhydrous ether, Distilled water, and Glassware (flasks, beakers, stirring rod)
Procedure
- Weigh out the desired amount of aluminum chloride and sodium laurate or potassium laurate in a 1:2 molar ratio. For example, if you want to prepare 1 gram of aluminum laurate, you will need 0.2 grams of aluminum chloride and 0.4 grams of sodium laurate or potassium laurate.
- Dissolve the aluminum chloride in anhydrous ether in a dry flask. Stir the mixture until the aluminum chloride is fully dissolved.
- Add the sodium laurate or potassium laurate to the flask and stir until the mixture is homogenous.
- Slowly add distilled water to the flask while stirring continuously. This will cause a white precipitate to form.
- Continue stirring the mixture for 30-60 minutes to ensure complete reaction.
- Filter the mixture to collect the white precipitate (aluminum laurate). Wash the precipitate with distilled water to remove any impurities.
- Dry the aluminum laurate in an oven at 50-60°C until it is completely dry.
Note: It is important to handle aluminum chloride with care as it is a highly reactive and corrosive substance. Make sure to wear appropriate personal protective equipment (gloves, goggles, lab coat) and work in a well-ventilated area.
Use
Aluminum laurate is used as an anticaking agent, free-flow agent, or emulsifier. It is used in the production of various products, such as cosmetics, personal care products, and pharmaceuticals. It is often used as a thickening agent, emulsifier, and stabilizer due to its ability to form gels and increase the viscosity of liquids. It is also used as an antiperspirant agent in some deodorant products.
It can also be used to increase the viscosity, or thickness, of a product. It has good stability and can help to prevent products from separating over time. It is considered to be a mild ingredient that is unlikely to cause irritation or allergic reactions. In addition to its use in personal care products, aluminum laurate is also used in the manufacture of plastics and as a lubricant in metalworking.
Safety issue
However, there are concerns about the safety of aluminum laurate, as aluminum has been linked to health problems such as breast cancer, Alzheimer’s disease, and neurological disorders. The use of aluminum laurate in cosmetic and personal care products is regulated by various agencies around the world, and its concentration in products is limited to ensure its safety.