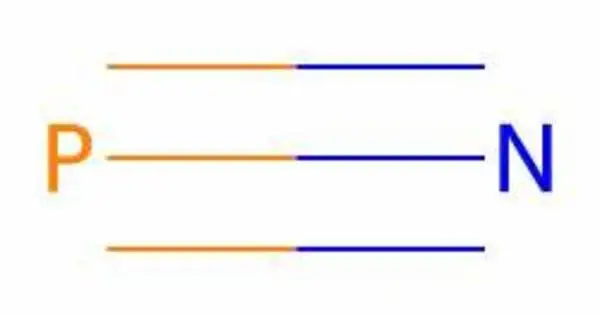
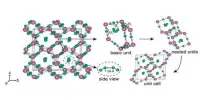
Phosphorus mononitride is an inorganic compound with the chemical formula PN. It is often denoted as P3N2, is a chemical compound composed of phosphorus and nitrogen. Containing only phosphorus and nitrogen, this material is classified as a binary nitride. Its molecular formula suggests that it contains three phosphorus atoms and two nitrogen atoms. PN is reactive and can participate in various chemical reactions, especially with other nitrogen and phosphorus compounds.
From the Lewis structure perspective, it can be represented with a P-N triple bond with a lone pair on each atom. It is isoelectronic with N2, CO, P2, CS and SiO. The compound is known for its interesting electronic and structural properties. It typically exists as a linear molecule, with a single phosphorus atom bonded to a nitrogen atom.
Properties
- Chemical Formula: PN
- Molecular Weight: Approximately 47.97 g/mol
- Melting and Boiling Points: It is known to have a melting point around -128 degrees Celsius and a boiling point of approximately -110 degrees Celsius.
- Magnetic Properties: It exhibits magnetic behavior due to unpaired electrons in its molecular orbitals.
- Occurrence: It is not commonly found in nature and is typically synthesized in laboratories.
Preparation
Phosphorus mononitride can be prepared under specific conditions, such as high-temperature reactions between phosphorus and nitrogen. It has been studied for its potential applications in electronic and optical devices. The compound exhibits semiconducting properties, making it of interest in the field of materials science.
The compound is highly unstable in standard conditions, tending to rapidly self polymerize. It can be isolated within argon and krypton matrices at 10 K (−263.1 °C). Due to its instability, documentation of reactions with other molecules is limited. Most of its reactivity has thus far been probed and studied at transition metal centers.
Phosphorus mononitride was the first identified phosphorus compound in the interstellar medium and is even thought to be an important molecule in the atmospheres of Jupiter and Saturn.
Applications
PN has applications in the field of semiconductor technology. It can be used as a precursor or dopant in the production of certain types of semiconductors.
Toxicity
Information about the toxicity of phosphorus mononitride is limited. As with many compounds, caution should be exercised when handling it, and appropriate safety measures should be followed.