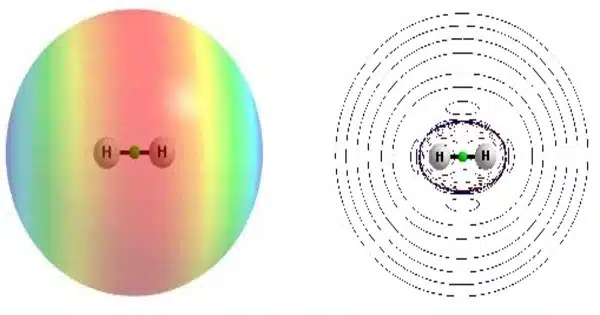
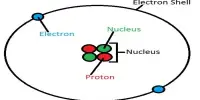
The dihydrogen cation, also known as the hydrogen molecular ion, is a positive ion with the formula H+2. It is made up of two hydrogen nuclei (protons) that share a single electron. It is the most basic molecular ion. The ion can be created by ionizing a neutral hydrogen molecule H2. It is commonly formed in space by the action of cosmic rays on molecular clouds.
It also known as the protonated hydrogen molecule or H2+, is a molecular ion composed of two hydrogen atoms plus one additional proton. Because it is formed by removing one electron from a hydrogen molecule (H2), it is denoted as H2+.
The dihydrogen cation is a highly reactive species that is involved in a variety of chemical reactions and processes. It is especially important in astrochemistry and the study of interstellar clouds. The dihydrogen cation is formed in these environments via ionization processes such as photoionization or charge transfer reactions.
The dihydrogen cation is of great historical and theoretical interest because, with only one electron, the quantum mechanics equations that describe its structure are relatively simple to deal with Ø. Burrau developed the first such solution in 1927, just one year after the wave theory of quantum mechanics was published.
Because of its simplicity, the dihydrogen cation serves as a fundamental example for understanding molecular ion bonding and properties. The H2+ ion is made up of two protons and one electron, giving it a net positive charge. A covalent bond holds the two protons together, and the electron is shared between them.
The dihydrogen cation has been thoroughly investigated using both experimental and theoretical methods. Its spectroscopic properties, including vibrational and rotational energy levels, have been measured, providing useful information for understanding molecular bonding and dynamics. Theoretical calculations, such as quantum mechanical models, are also used to investigate the H2+ ion’s behavior and interactions with other molecules.
Overall, the dihydrogen cation is an important species in chemistry and astrophysics, helping us understand molecular structure, chemical reactions, and the properties of ionized molecules in different environments.