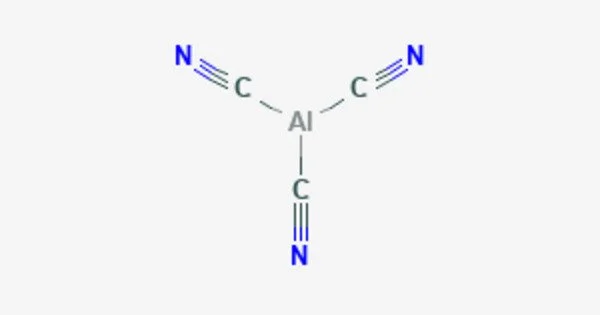
Aluminum cyanide is a chemical compound with the formula Al(CN)3. It is a metallic cyanide. It is a white, crystalline solid that is soluble in water and releases hydrogen cyanide gas upon exposure to moisture. The compound is used as a reducing agent in the synthesis of aluminum compounds, as well as in the purification of aluminum. It is highly toxic and should be handled with caution.
Aluminium cyanide was created as an ammoniate by reacting aluminium metal with mercuric cyanide in liquid ammonia. Water reacts with aluminium cyanide to form aluminium hydroxide. Cyanide is a fast-acting, potentially lethal chemical that can exist as both a gas and a crystalline salt. In high enough concentrations, either form can be lethal. Some people can detect the distinctive ‘bitter almond’ smell of cyanide, but this is not a reliable indicator because it does not always emit it, and not everyone can detect it.
Properties
- Chemical formula: C3AlN3
- Molar mass: 105.036 g·mol−1
The inorganic compound ammonium cyanide (NH4CN) is an unstable inorganic chemical. In organic synthesis, ammonium cyanide is commonly employed. It cannot be exported or sold commercially due to its instability.
Production
Cyanide is a chemical compound with the CN group. To form the cyano group, a carbon atom is triple-bonded to a nitrogen atom. The cyanide group is present in inorganic cyanides as the anion CN. Sodium cyanide and potassium cyanide are extremely lethal soluble salts. The highly flammable liquid hydrocyanic acid, also known as hydrogen cyanide or HCN, is widely used in industry. It is made from acidified cyanide salts.
Cyanide salts are primarily used in electroplating, metallurgy, the production of organic chemicals (acrylonitrile, methyl methacrylate, adiponitrile), photographic development, gold and silver extraction from ores, tanning leather, and the manufacture of plastics and fibers. They’re also used in the production of fumigation chemicals, insecticides, and rodenticides.
Certain bacteria, fungi, and algae produce cyanides. It acts as an antifeedant in a variety of plants. Cyanides are abundant in certain seeds and fruit stones, such as bitter almonds, apricots, apples, and peaches. Cyanogenic compounds are chemical compounds that can release cyanide. Cyanides are typically bound to sugar molecules in plants as cyanogenic glycosides, which protect the plant from herbivores. Cassava roots, an important potato-like food grown in tropical countries (and the basis for tapioca), contain cyanogenic glycosides as well.