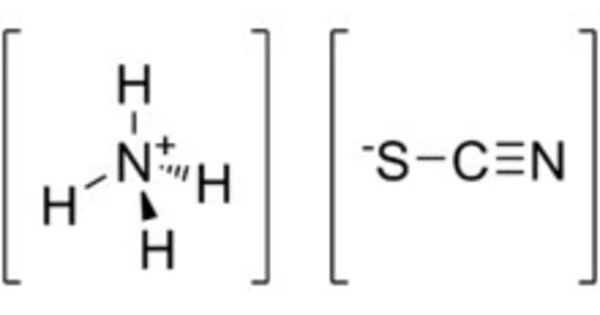Ammonium thiocyanate has the formula NH4SCN and is an inorganic compound. It is the ammonium cation and thiocyanate anion salt. It is a crystalline solid with no color. It dissolves in water. The primary risk is the threat to the environment. It is used in chemical analysis, photography, as a fertilizer, and a variety of other applications.
It is used in the production of herbicides, thiourea, and transparent artificial resins, as a stabilizing agent in photography, as an adjuvant in textile dyeing and printing, as a tracer in oil fields, in the separation of hafnium from zirconium, and in titrimetric analyses.
Properties
Ammonium thiocyanate is a colorless lustrous monoclinic flaky or columnar crystal and has a molecular weight of 76.12. At 92 ℃, it is a rhombohedral crystal with a melting point of 147 ℃ (decompose at 170 ℃), a relative density of 1.3057, and a refractive index of 1.685. It is easily soluble in water and will have an endothermic reaction when dissolved in water.
- Chemical formula: NH4SCN
- Molar mass: 76.122 g/mol
- Appearance: Colorless hygroscopic crystalline solid
- Density: 1.305 g/cm3
- Melting point: 149.5 °C (301.1 °F; 422.6 K)
- Boiling point: 170 °C (338 °F; 443 K) (decomposes)
- Solubility in water: 128 g/100 mL (0 °C)
- Solubility: soluble in liquid ammonia, alcohol, acetone
Preparation
Carbon disulfide reacts with aqueous ammonia to produce ammonium thiocyanate. As an intermediate in this reaction, ammonium dithiocarbamate is formed, which upon heating decomposes to ammonium thiocyanate and hydrogen sulfide:
CS2 + 2 NH3(aq) → NH2C(=S)SNH4 → NH4SCN + H2S
Alcohol, alkali metal hydroxide, acetone, pyridine, and liquid sulfur dioxide are all soluble in it, but chloroform is not. When exposed to sunlight, its solution turns red. In the case of ferric iron, it produces iron thiocyanate of blood red color, which can be quantified colorimetrically based on the color of the standard solution. When heated to 140 °C, it can produce Thiourea, but at 176 °C, it decomposes into ammonia, hydrogen sulfide, and carbon disulfide.
Reactions
Ammonium thiocyanate is stable in air, but it isomerizes to thiourea when heated:
At 150 °C and 180 °C, the equilibrium mixtures contain 30.3 percent and 25.3 percent (by weight) thiourea, respectively. The dry powder decomposes to ammonia, hydrogen sulfide, and carbon disulfide when heated to 200 °C, leaving a residue of guanidinium thiocyanate.
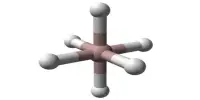
Because of the ammonium ion, NH4SCN is weakly acidic; it reacts with alkali hydroxides, such as sodium hydroxide or potassium hydroxide, to form sodium thiocyanate or potassium thiocyanate, along with water and ammonia. Specifically, the thiocyanate anion reacts with ferric salts to form a deep-red ferric thiocyanate complex:
6 SCN− + Fe3+ → [Fe(SCN)6]3−
Ammonium thiocyanate reacts with several metal ions including copper, silver, zinc, lead, and mercury, forming their thiocyanate precipitates, which may be extracted into organic solvents.
Uses
Ammonium thiocyanate is used in the production of herbicides, thiourea, and transparent artificial resins; in matches; as a stabilizing agent in photography; in various rustproofing compositions; as an adjuvant in textile dyeing and printing; as an adjuvant in textile dyeing and printing; as a tracer in oil fields; in the separation of hafnium from zirconium (important for the production of hafnium-free zircallo
Colorimetry can also be used to determine the iron content of soft drinks using ammonium thiocyanate. It can also be used to separate quinidine from liquors after quinine has been isolated from the neutral, aqueous sulphate solution.