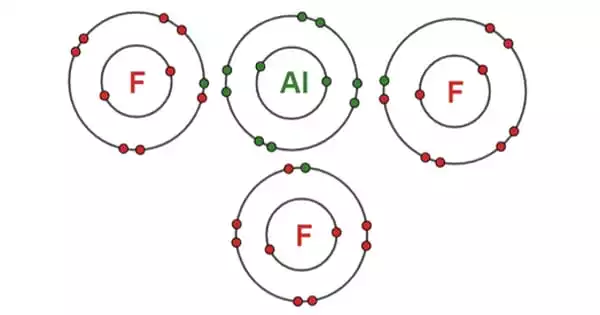
Inorganic compounds with the formula AlF3•xH2O are referred to as aluminium fluoride. All of them are colorless solids. Aluminium metal is produced using anhydrous AlF3. Several are found as minerals. It takes the form of odorless white powder or granules. It can be found in nature as oskarssonite and rosenbergite. It can also be produced synthetically. The vast bulk of aluminium fluoride is created by heating hydrogen fluoride with alumina or aluminium oxide to 700 °C.
Aluminum fluoride is widely utilized in ceramics, where it is the major chemical compound in finished product glazes. It is also used in the production of refractory ceramic ceramics.
Properties
Aluminum fluoride is a white crystal that sublimes (760 mm) at 1272 degrees Celsius. It has a density of 2.882 and is a powerful tissue irritant. Aluminum fluoride, Trihydrate is water-soluble but insoluble in most organic solvents, whereas Aluminum fluoride, Anhydrous is water-insoluble.
- Appearance: Hygroscopic white or colorless solid
- Melting Point: 1,291 °C (anhydrous)
- Molar Mass: 83.977 g/mol (anhydrous); 101.992 g/mol (monohydrate); 138.023 g/mol (trihydrate)
- Density: 3.10 g/cm3 (anhydrous); 2.17 g/cm3 (monohydrate); 3.10 g/cm3 (trihydrate)
- Solubility in Water: Soluble in water

Structure
Anhydrous AlF3 adopts the rhenium trioxide pattern, with deformed AlF6 octahedra, according to X-ray crystallography. Each fluoride has two Al centers attached to it. AlF3 has a high melting point due to its three-dimensional polymeric structure. In the solid-state, the other trihalides of aluminum have different structures: AlCl6 has a layer structure, whilst AlBr3 and AlI3 are molecular dimers. They also have low melting temperatures and easily evaporate to form dimers. Aluminium fluoride exists in the gas phase as trigonal molecules with D3h symmetry. This gaseous molecule’s Al–F bond lengths are 163 pm.
Occurrence and production
Aside from anhydrous AlF3, several hydrates are known. With the formula AlF3•xH2O, these compounds include monohydrate (x = 1), two polymorphs of the trihydrate (x = 3), and a hexahydrate (x = 6), and a nonahydrate (x = 9).
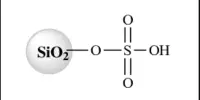
The majority of aluminium fluoride is produced by treating alumina with hydrogen fluoride at 700 °C: Fluorosilicic acid may also be used to make aluminum fluoride.
H2SiF6 + Al2O3 + 3 H2O → 2 AlF3 + SiO2 + 4 H2O
Alternatively, it is manufactured by thermal decomposition of ammonium hexafluoroaluminate. For small-scale laboratory preparations, AlF3 can also be prepared by treating aluminium hydroxide or aluminium metal with hydrogen fluoride.
Aluminium fluoride trihydrate is found in nature as the rare mineral rosenbergite. The anhydrous form appears as the relatively recently (as of 2020) recognized mineral óskarssonite. A related, exceedingly rare mineral, is zharchikhite, Al(OH)2F.
Applications
Aluminium fluoride is a critical additive in the electrolysis production of aluminum. Aluminum fluoride complexes are used in biology to research the mechanistic features of phosphoryl transfer processes, which are critical to cells because phosphoric acid anhydrides such as ATP and GTP govern the majority of the activities involved in metabolism, growth, and differentiation. Common Uses are –
• Used as an additive for the production of aluminium by electrolysis
• Used in the fermentation process in beer and wine factories
• Used in the production of low index optical thin film
• Used as a catalyst in the organic compound synthesis process