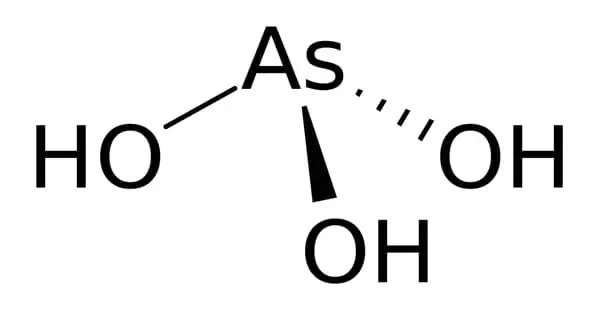
Arsenic acid, with the formula H3AsO3, is an inorganic chemical. Although it is known to occur in aqueous solutions, it has not been isolated as a pure substance, which does not diminish the relevance of As(OH)3. It is an arsenic oxoacid made up of three hydroxy groups linked to a central arsenic atom. Compounds containing arsenic are extremely poisonous and carcinogenic. Arsenic trioxide, the anhydride form of arsenous acid, is employed as a herbicide, insecticide, and rodenticide.
Properties
It is a clear or white glass, amorphous block, or crystalline powder. It is a type of amphoteric oxide. It has a melting point of 275 degrees Celsius and a boiling point of 465 degrees Celsius. It is dissolved in a solution of weak hydrochloric acid, sodium hydroxide, and sodium carbonate. It is mildly soluble in cold water, marginally soluble in boiling water, and completely insoluble in chloroform, ethanol, and ether.
- Chemical formula: H3AsO3
- Molar mass: 125.94 g/mol
- Appearance: Only exists in aqueous solutions
- Conjugate base: Arsenite
Synthesis
The preparation of As(OH)3 involves slow hydrolysis of arsenic trioxide in water. Addition of base converts arsenous acid to the arsenite ions [AsO(OH)2]−, [AsO2(OH)]2−, and [AsO3]3−.
As(OH)3 is a pyramidal molecule made up of three hydroxyl groups that are bound to arsenic. The 1H NMR spectrum of arsenic acid solutions is composed of a single signal, which corresponds to the molecule’s great symmetry. HPO(OH)2 is the structure of the presumably related phosphorous acid H3PO3. Arsenic acid’s structural counterpart (P(OH)3) is a very tiny equilibrium component of such solutions. The different behavior of the As and P compounds follow a tendency in which lighter members of the main group elements have higher oxidation states than their heavier congeners.
Reactions
With its first pKa being 9.2, As(OH)3 is a weak acid. Reactions attributed to aqueous arsenic trioxide are due to arsenous acid and its conjugate bases.
Like arsenic trioxide, arsenous acid is sometimes amphoteric. For example, it reacts with hydrochloric, hydrobromic, and hydroiodic acids to produce arsenic trichloride, tribromide, and triiodide.
As(OH)3 + 3 HCl ⇌ AsCl3 + 3 H2O
As(OH)3 + 3 HBr ⇌ AsBr3 + 3 H2O
As(OH)3 + 3 HI ⇌ AsI3 + 3 H2O
Reaction of arsenous acid with methyl iodide gives methylarsonic acid. This historically significant conversion is the Meyer reaction:
As(OH)3 + CH3I + NaOH ⇌ CH3AsO(OH)2 + NaI + H2O
Alkylation occurs at arsenic, and the oxidation state of arsenic increases from +3 to +5.
Toxicology
Arsenic containing compounds are highly toxic and carcinogenic. The anhydride form of arsenous acid, arsenic trioxide, is used as a herbicide, pesticide, and rodenticide.